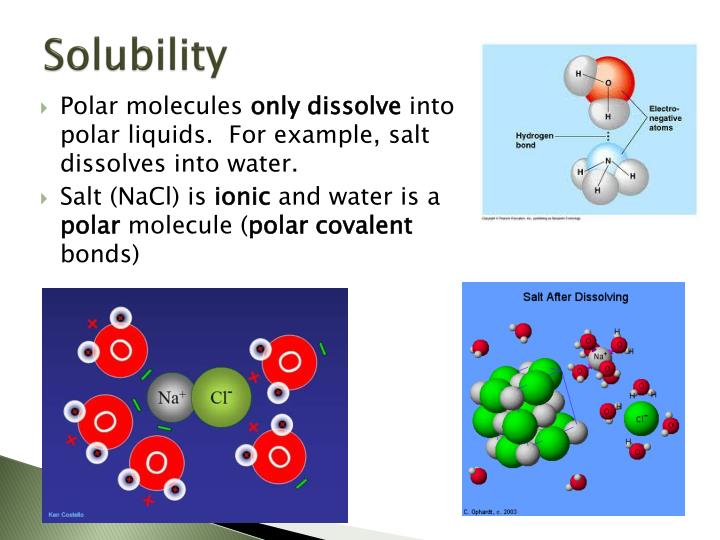
Why Are Ionic Compounds Water Soluble . although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a overview of solubility as a key property of ionic compounds,. predict solubilities of ionic compounds in water using the solubility rules.
from www.slideserve.com
ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. The extent to which a substance. predict solubilities of ionic compounds in water using the solubility rules. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a overview of solubility as a key property of ionic compounds,.
PPT Bonding PowerPoint Presentation ID3050946
Why Are Ionic Compounds Water Soluble a overview of solubility as a key property of ionic compounds,. The extent to which a substance. a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds in water using the solubility rules.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID7002512 Why Are Ionic Compounds Water Soluble ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a overview of solubility as a key property of ionic compounds,. predict solubilities of ionic compounds in water using the solubility rules. when ionic bonds form, one atom becomes positively. Why Are Ionic Compounds Water Soluble.
From ar.inspiredpencil.com
Solubility In Water Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. ionic compounds dissolve in water if the energy given off when. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Presentation ID643607 Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance. a overview of solubility as a key property of ionic compounds,. ionic compounds dissolve in water if the energy given. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download ID6037545 Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. . Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is completely dissolved into aqueous Why Are Ionic Compounds Water Soluble ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds in water using the solubility rules. a overview of solubility as a key property of ionic compounds,. The extent to which a substance. when ionic. Why Are Ionic Compounds Water Soluble.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free download ID1820946 Why Are Ionic Compounds Water Soluble The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. ionic compounds dissolve in water if. Why Are Ionic Compounds Water Soluble.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. ionic compounds dissolve in water if. Why Are Ionic Compounds Water Soluble.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. The extent to which a substance. although the charge on the ion remains the same as we move down the. Why Are Ionic Compounds Water Soluble.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Water Soluble.
From www.youtube.com
why ionic compounds are soluble in water? ( A proper reason ) YouTube Why Are Ionic Compounds Water Soluble a overview of solubility as a key property of ionic compounds,. The extent to which a substance. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is completely dissolved into aqueous Why Are Ionic Compounds Water Soluble The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. a overview of solubility as a key property of ionic compounds,. although the charge on the ion remains the same as we move down the group, the ionic radius increases so. Why Are Ionic Compounds Water Soluble.
From quizlet.com
Solubility Rules for Ionic Compounds Diagram Quizlet Why Are Ionic Compounds Water Soluble a overview of solubility as a key property of ionic compounds,. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the. Why Are Ionic Compounds Water Soluble.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the. Why Are Ionic Compounds Water Soluble.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Compounds AII sodium (Na Why Are Ionic Compounds Water Soluble The extent to which a substance. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. when ionic. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance. a overview of solubility as a key property of ionic compounds,. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation ID3205890 Why Are Ionic Compounds Water Soluble although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic bonds form, one. Why Are Ionic Compounds Water Soluble.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Why Are Ionic Compounds Water Soluble predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. . Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID2871797 Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. a overview of solubility as a key property of ionic compounds,. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. although the charge on the ion remains the. Why Are Ionic Compounds Water Soluble.
From app.jove.com
Solubility Properties of Ionic Compounds Concept Chemistry JoVe Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. The extent to which a substance. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. although the charge on the. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download ID3076492 Why Are Ionic Compounds Water Soluble The extent to which a substance. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a overview of solubility as a key property of ionic compounds,. although the charge on the ion remains the same as we move down the. Why Are Ionic Compounds Water Soluble.
From www.youtube.com
Using Solubility Rules to Predict Solubility of Ionic Compounds YouTube Why Are Ionic Compounds Water Soluble although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. when ionic bonds form, one atom becomes positively charged (+), and. Why Are Ionic Compounds Water Soluble.
From www.chegg.com
Solved Solubility rules for ionic compounds in water are Why Are Ionic Compounds Water Soluble ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in. Why Are Ionic Compounds Water Soluble.
From www.numerade.com
SOLVEDWhy are the ionic compounds soluble in water? Why Are Ionic Compounds Water Soluble although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Compounds Water Soluble predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. a overview of solubility as a key property of ionic compounds,. when ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Water Soluble.
From kunduz.com
[ANSWERED] Which of the following ionic compounds is soluble in water? Kunduz Why Are Ionic Compounds Water Soluble although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds. Why Are Ionic Compounds Water Soluble.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the. Why Are Ionic Compounds Water Soluble.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility chart given in the lab Why Are Ionic Compounds Water Soluble a overview of solubility as a key property of ionic compounds,. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions PowerPoint Presentation ID Why Are Ionic Compounds Water Soluble when ionic bonds form, one atom becomes positively charged (+), and the other one becomes negatively charged. The extent to which a substance. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. predict solubilities of ionic compounds in water using. Why Are Ionic Compounds Water Soluble.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Water Soluble The extent to which a substance. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Stoichiometry PowerPoint Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. although the charge on the ion remains the same as we move down the group, the ionic radius increases so the charge density of the ion. when ionic compounds dissolve in water, the ions in the solid separate. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is completely dissolved into aqueous Why Are Ionic Compounds Water Soluble when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the ions in the solid. Why Are Ionic Compounds Water Soluble.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Why Are Ionic Compounds Water Soluble predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a overview of solubility as a key property of ionic compounds,. when ionic bonds form, one atom becomes positively charged (+), and the other one becomes. Why Are Ionic Compounds Water Soluble.