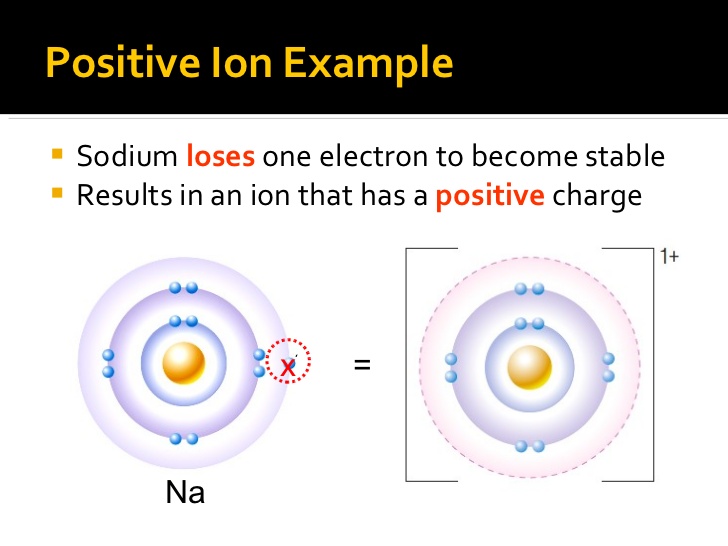
Does Ba Form A Positive Ion . Metals are found on the left hand side of the periodic table, they always form positive ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. The magnitude of the positive charge is the same. The ions are positive, because they have more. Barium is the 56th element in the periodic table and the symbol is ‘ba’. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions (cations). These ions are positive because they contain more protons. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive.
from socratic.org
The magnitude of the positive charge is the same. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Metals form positive ions (cations). Barium is the 56th element in the periodic table and the symbol is ‘ba’. The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Metal atoms lose electrons from their outer shell when they form ions: Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus.
What happens if an atom is not neutral? Socratic
Does Ba Form A Positive Ion The ions are positive, because they have more. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Barium is the 56th element in the periodic table and the symbol is ‘ba’. These ions are positive because they contain more protons. The ions are positive, because they have more. The magnitude of the positive charge is the same. Metals form positive ions (cations). Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Metal atoms lose electrons from their outer shell when they form ions: Metals are found on the left hand side of the periodic table, they always form positive ions.
From www.slideserve.com
PPT Chemical Bonding PART 1 IONIC PowerPoint Presentation, free Does Ba Form A Positive Ion These ions are positive because they contain more protons. Barium is the 56th element in the periodic table and the symbol is ‘ba’. The magnitude of the positive charge is the same. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The. Does Ba Form A Positive Ion.
From design.udlvirtual.edu.pe
What Does It Mean When An Ion Has A Positive Charge Design Talk Does Ba Form A Positive Ion Barium is the 56th element in the periodic table and the symbol is ‘ba’. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. The ions are positive, because they have more. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Does Ba Form A Positive Ion.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Does Ba Form A Positive Ion Metals form positive ions (cations). The ions are positive, because they have more. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to. Does Ba Form A Positive Ion.
From www.slideshare.net
Ch 8 ionic compounds Does Ba Form A Positive Ion Barium is the 56th element in the periodic table and the symbol is ‘ba’. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when.. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Does Ba Form A Positive Ion Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Metals are found on the left hand side of the periodic table, they always form positive ions. Metals form positive ions (cations). A magnesium atom. Does Ba Form A Positive Ion.
From slideplayer.com
Chapter 6 Section 3 Periodic Trends. ppt download Does Ba Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Metals are found on the left hand side of the periodic table,. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT Ions PowerPoint Presentation, free download ID6738771 Does Ba Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Metals are found on the left hand side of the periodic table, they always form positive ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A magnesium atom must. Does Ba Form A Positive Ion.
From socratic.org
What happens if an atom is not neutral? Socratic Does Ba Form A Positive Ion Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms. Does Ba Form A Positive Ion.
From www.nagwa.com
Question Video Describing the Attraction between the Ions of an Ionic Does Ba Form A Positive Ion Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. The ions are positive, because they have more. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons. Does Ba Form A Positive Ion.
From learningschooloviducts.z14.web.core.windows.net
Teaching How Ions Are Formed Does Ba Form A Positive Ion Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms. Does Ba Form A Positive Ion.
From quizzfullmarks77.z19.web.core.windows.net
Write The Differences Between Atoms And Ions Does Ba Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. These ions are positive because they contain more protons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Barium is the 56th element. Does Ba Form A Positive Ion.
From www.breakingatom.com
Ions of Elements Does Ba Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Metals form positive ions (cations). Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Barium has an atomic number of 56, which means that its atom has 56 electrons around its. Does Ba Form A Positive Ion.
From soraivasilva29.blogspot.com
Ba(No3)2 Positive And Negative Ion Solved Data Observations And Does Ba Form A Positive Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Barium is the 56th element in the periodic table and the symbol is ‘ba’. The ions are positive, because they have more. These ions are positive because they contain more protons. Soluble chromates react with barium ion to form a finely. Does Ba Form A Positive Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Does Ba Form A Positive Ion Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. The magnitude of the positive charge is the same. These ions are positive because they contain more protons. Metals are found on the left hand side of the periodic table, they always form positive ions. Forming positive ions (cations) atoms lose electrons. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Does Ba Form A Positive Ion Metals form positive ions (cations). Metal atoms lose electrons from their outer shell when they form ions: The magnitude of the positive charge is the same. Metals are found on the left hand side of the periodic table, they always form positive ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons. Does Ba Form A Positive Ion.
From shaniaferscosta.blogspot.com
Describe the Formation of Positive and Negative Ions Does Ba Form A Positive Ion Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. These ions are positive because they contain more protons. Metal atoms lose electrons from their outer shell when they form ions: The ions are positive, because they have more. Metals are found on the left hand side of the periodic table, they always. Does Ba Form A Positive Ion.
From studylibrarybeverly.z21.web.core.windows.net
How Do Positive And Negative Ions Form Does Ba Form A Positive Ion Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Atoms that lose electrons acquire a positive charge. Does Ba Form A Positive Ion.
From www.youtube.com
Formation of positive ion YouTube Does Ba Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Metals form positive ions (cations). Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Barium is the 56th element in the periodic table and the. Does Ba Form A Positive Ion.
From www.nagwa.com
Lesson Video Ions Nagwa Does Ba Form A Positive Ion Metals are found on the left hand side of the periodic table, they always form positive ions. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. The ions are positive, because they have more. Metal atoms lose electrons from their outer shell when they form ions: The magnitude of the positive charge. Does Ba Form A Positive Ion.
From www.nagwa.com
Question Video Identifying Which Element Can Form a Positive Ion in an Does Ba Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Metals are. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Does Ba Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Metals form positive ions (cations). Metals are found on the left hand side of the periodic. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT 10 Write formulas for compounds formed from these pairs of ions Does Ba Form A Positive Ion Metals form positive ions (cations). The ions are positive, because they have more. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Metal atoms lose electrons from their outer shell when they form ions: Metals are found on the left hand side of the periodic table, they always form positive ions. Soluble. Does Ba Form A Positive Ion.
From worksheetzonejimply.z5.web.core.windows.net
Chemistry Positive And Negative Ions Does Ba Form A Positive Ion These ions are positive because they contain more protons. The ions are positive, because they have more. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. The magnitude of the positive charge is the same. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Barium. Does Ba Form A Positive Ion.
From learninglibraryhaney.z19.web.core.windows.net
Explain The Formation Of Ions Does Ba Form A Positive Ion Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Metal atoms lose electrons from their outer shell when they form ions: Metals are found on the left hand side of the periodic table, they always form positive ions. The magnitude of the positive charge is the same. A cation (a positive ion). Does Ba Form A Positive Ion.
From www.youtube.com
Ba 2+ Electron Configuration (Barium Ion) YouTube Does Ba Form A Positive Ion Barium is the 56th element in the periodic table and the symbol is ‘ba’. The magnitude of the positive charge is the same. Metals are found on the left hand side of the periodic table, they always form positive ions. The ions are positive, because they have more. Barium has an atomic number of 56, which means that its atom. Does Ba Form A Positive Ion.
From spmkimia.onlinetuition.com.my
Pembentukan Ion Positif Nota Ulangkaji Kimia SPM Tingkatan 4/Tingkatan 5 Does Ba Form A Positive Ion Metals are found on the left hand side of the periodic table, they always form positive ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same. Does Ba Form A Positive Ion.
From www.savemyexams.com
Formation of Ions Edexcel IGCSE Chemistry Revision Notes 2019 Does Ba Form A Positive Ion Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. The ions are positive, because they have more. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Does Ba Form A Positive Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Does Ba Form A Positive Ion Barium is the 56th element in the periodic table and the symbol is ‘ba’. These ions are positive because they contain more protons. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called. Metals are found on the left hand side of the periodic table, they always form positive ions. Metals form positive. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Ba Form A Positive Ion Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Forming positive ions (cations) atoms lose electrons from their outer shell when they form positive. Does Ba Form A Positive Ion.
From lessonschoolquintuples.z5.web.core.windows.net
How To Ions Form Does Ba Form A Positive Ion The magnitude of the positive charge is the same. Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions (cations). Metals are found on the left hand side of the periodic table, they always form positive ions. These ions are positive because they contain more protons. A magnesium atom must lose two electrons to. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Ba Form A Positive Ion Metals form positive ions (cations). Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. These ions are positive because they contain more protons. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Metal atoms lose electrons from their outer shell when they form ions:. Does Ba Form A Positive Ion.
From slideplayer.com
Ions, Valences and Oxidation Numbers ppt download Does Ba Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Metals form positive ions (cations). The ions are positive, because they have more. Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Barium is the 56th element in the periodic. Does Ba Form A Positive Ion.
From www.slideserve.com
PPT The History of the Modern Periodic Table PowerPoint Presentation Does Ba Form A Positive Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Soluble chromates react with barium ion to form a finely divided yellow precipitate of barium chromate: Forming positive ions (cations). Does Ba Form A Positive Ion.
From chem.libretexts.org
3.4 Ionic Nomenclature Chemistry LibreTexts Does Ba Form A Positive Ion Metals form positive ions (cations). Metal atoms lose electrons from their outer shell when they form ions: These ions are positive because they contain more protons. The magnitude of the positive charge is the same. Barium has an atomic number of 56, which means that its atom has 56 electrons around its nucleus. Barium is the 56th element in the. Does Ba Form A Positive Ion.
From www.nagwa.com
Question Video Identifying What Changes When an Atom Forms a Positive Does Ba Form A Positive Ion Metals are found on the left hand side of the periodic table, they always form positive ions. The magnitude of the positive charge is the same. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Barium has an atomic number of 56, which means that its atom has 56 electrons. Does Ba Form A Positive Ion.