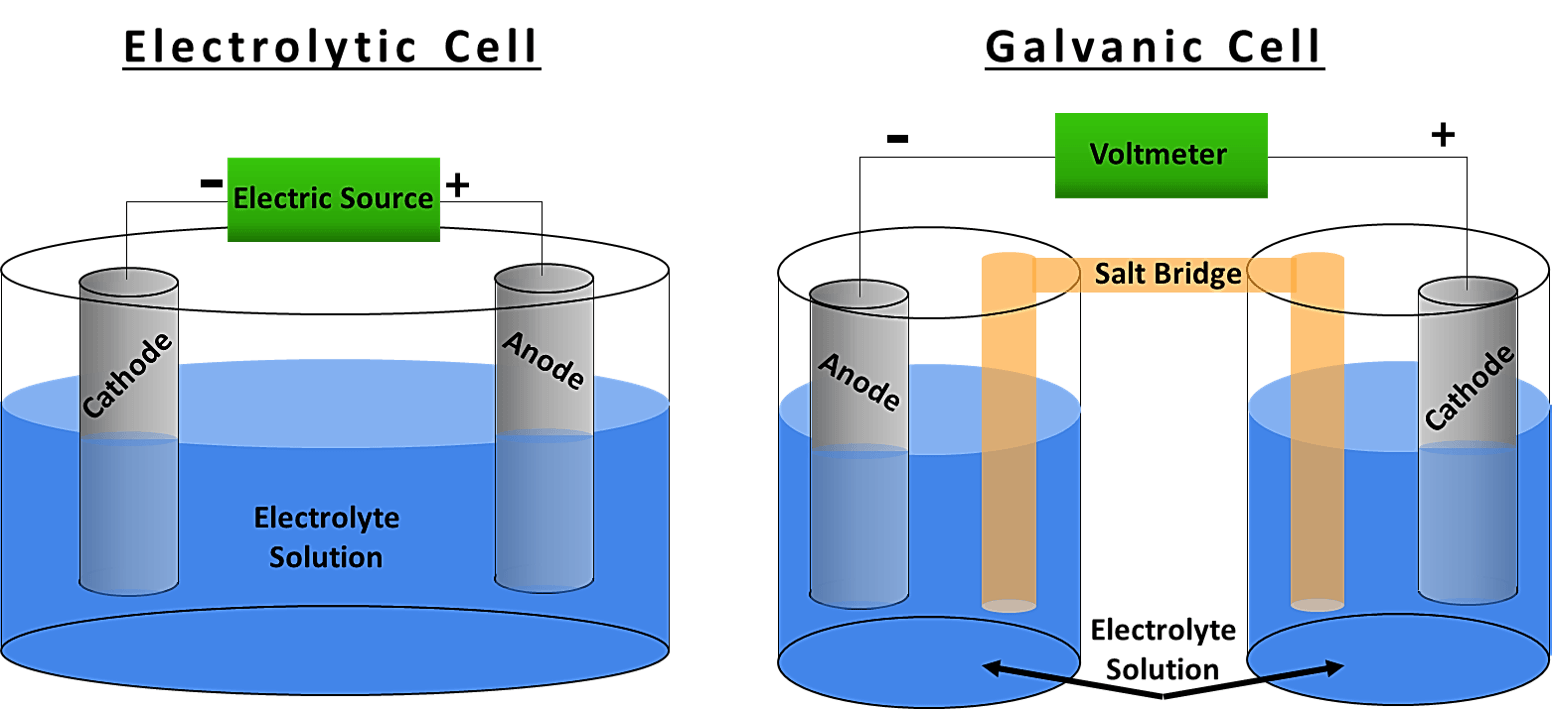
Electrochemistry Electrochemical Cells . An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. These devices are capable of converting chemical energy into electrical energy, or vice versa. Describe the basic components of electrochemical cells. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. List some of the characteristics, applications and limitations of cells and batteries. Define electrolysis and list several of its applications. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Know the difference between galvanic and electrolytic cells.
from www.expii.com
This kind of cell includes the galvanic, or voltaic, cell, named after. List some of the characteristics, applications and limitations of cells and batteries. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. These devices are capable of converting chemical energy into electrical energy, or vice versa. Know the difference between galvanic and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it.
Electrochemical Cell — Definition & Overview Expii
Electrochemistry Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. List some of the characteristics, applications and limitations of cells and batteries. Describe the basic components of electrochemical cells. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. Define electrolysis and list several of its applications. Know the difference between galvanic and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. These devices are capable of converting chemical energy into electrical energy, or vice versa.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemistry Electrochemical Cells Define electrolysis and list several of its applications. Describe the basic components of electrochemical cells. Know the difference between galvanic and electrolytic cells. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell splits the. Electrochemistry Electrochemical Cells.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Electrochemistry Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a device that can generate electrical energy from. Electrochemistry Electrochemical Cells.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell Electrochemistry Electrochemical Cells List some of the characteristics, applications and limitations of cells and batteries. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Describe the. Electrochemistry Electrochemical Cells.
From saylordotorg.github.io
Electrochemistry Electrochemistry Electrochemical Cells Know the difference between galvanic and electrolytic cells. Define electrolysis and list several of its applications. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox. Electrochemistry Electrochemical Cells.
From learningkurugon1.z22.web.core.windows.net
Electrochemical Cells Gcse Chemistry Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. This kind of cell includes the galvanic, or voltaic, cell, named after. Describe the basic components of electrochemical cells. These devices are capable of converting chemical energy into electrical energy, or vice versa. An. Electrochemistry Electrochemical Cells.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. These devices are capable of converting. Electrochemistry Electrochemical Cells.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Electrochemical Cells List some of the characteristics, applications and limitations of cells and batteries. Know the difference between galvanic and electrolytic cells. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a device that can generate electrical energy from the chemical. Electrochemistry Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemistry Electrochemical Cells Define electrolysis and list several of its applications. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. This kind of cell. Electrochemistry Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemistry Electrochemical Cells Describe the basic components of electrochemical cells. These devices are capable of converting chemical energy into electrical energy, or vice versa. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. Define electrolysis and list several of its applications. List some of the characteristics,. Electrochemistry Electrochemical Cells.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Define electrolysis and list several of its applications. An. Electrochemistry Electrochemical Cells.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. These devices are capable of converting chemical energy into electrical energy, or vice versa. Know the difference between galvanic and electrolytic cells. List some of the characteristics, applications and limitations of cells and batteries.. Electrochemistry Electrochemical Cells.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ Electrochemistry Electrochemical Cells An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These devices are capable of converting chemical energy into electrical energy, or vice versa. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. In electrochemistry the term “cell” is commonly used for a wide variety. Electrochemistry Electrochemical Cells.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemistry Electrochemical Cells Know the difference between galvanic and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. These devices are capable of converting chemical energy into electrical energy, or vice versa. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring. Electrochemistry Electrochemical Cells.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. Define electrolysis and list several of its applications. Know the difference between galvanic and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of. Electrochemistry Electrochemical Cells.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. List some of the characteristics, applications. Electrochemistry Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemistry Electrochemical Cells Know the difference between galvanic and electrolytic cells. This kind of cell includes the galvanic, or voltaic, cell, named after. Define electrolysis and list several of its applications. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous. Electrochemistry Electrochemical Cells.
From printablelibmolines.z13.web.core.windows.net
Diagram Of Electrochemical Cell Electrochemistry Electrochemical Cells Define electrolysis and list several of its applications. These devices are capable of converting chemical energy into electrical energy, or vice versa. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. Know the difference between galvanic and. Electrochemistry Electrochemical Cells.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. List some of the characteristics, applications and limitations of cells and batteries. These devices are capable of converting chemical energy into electrical energy, or vice versa. Know the difference between galvanic and electrolytic cells.. Electrochemistry Electrochemical Cells.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material Electrochemistry Electrochemical Cells These devices are capable of converting chemical energy into electrical energy, or vice versa. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell is a device that produces an electric current from energy released. Electrochemistry Electrochemical Cells.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemistry Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Describe the basic components of electrochemical cells. List some of the characteristics, applications and limitations of cells and batteries. Define electrolysis and list. Electrochemistry Electrochemical Cells.
From www.slideshare.net
Electrochemistry electrochemical cells Electrochemistry Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell is a device that produces an electric current from energy released by a. Electrochemistry Electrochemical Cells.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Electrochemical Cells An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These devices are capable of converting chemical energy into electrical energy, or vice versa. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a. Electrochemistry Electrochemical Cells.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Electrochemistry Electrochemical Cells Know the difference between galvanic and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Define electrolysis and list several of its applications. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a. Electrochemistry Electrochemical Cells.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemistry Electrochemical Cells List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Define electrolysis and list several of its applications. Know the difference between galvanic and electrolytic cells. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in. Electrochemistry Electrochemical Cells.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. Describe the basic components of electrochemical cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity. Electrochemistry Electrochemical Cells.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell Electrochemistry Electrochemical Cells Know the difference between galvanic and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. These devices are capable of converting chemical energy into electrical energy, or vice versa. List some of the characteristics, applications and limitations of cells and batteries. Define electrolysis and list. Electrochemistry Electrochemical Cells.
From saylordotorg.github.io
Electrochemistry Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. This kind of cell includes the galvanic, or voltaic, cell, named after. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different. Electrochemistry Electrochemical Cells.
From mavink.com
Components Of Electrochemical Cell Electrochemistry Electrochemical Cells An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. This kind of cell includes the galvanic, or voltaic, cell, named after. Describe the basic components of electrochemical cells.. Electrochemistry Electrochemical Cells.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Electrochemistry Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a device that produces an electric current from. Electrochemistry Electrochemical Cells.
From mavink.com
Electrochemical Cell Diagram Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of. Electrochemistry Electrochemical Cells.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemistry Electrochemical Cells An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic,. Electrochemistry Electrochemical Cells.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrochemistry Electrochemical Cells An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These devices are capable of converting chemical energy into electrical energy, or vice versa. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that produces an. Electrochemistry Electrochemical Cells.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemistry Electrochemical Cells In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. Know the. Electrochemistry Electrochemical Cells.
From www.chemistrystudent.com
Electrochemical Cells (ALevel) ChemistryStudent Electrochemistry Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after. Know the difference between galvanic and electrolytic cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. Describe the basic components of electrochemical cells. In electrochemistry. Electrochemistry Electrochemical Cells.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Electrochemistry Electrochemical Cells These devices are capable of converting chemical energy into electrical energy, or vice versa. In electrochemistry the term “cell” is commonly used for a wide variety of devices with different functions, shapes, and sizes in which electrochemical reactions take place. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Describe the basic components of. Electrochemistry Electrochemical Cells.