Device Listing Number Format . (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Download listing and proprietary name information through excel files that may be saved and searched locally. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. Then contract manufacturer or sterilizer may list • for combination products,. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. • manufacturer or specification developer must list device first;
from isgp.hik-proconnect.com
I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Releasable establishment registration and listing information under the freedom of information act is available by. Then contract manufacturer or sterilizer may list • for combination products,. Download listing and proprietary name information through excel files that may be saved and searched locally. • manufacturer or specification developer must list device first; In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin.
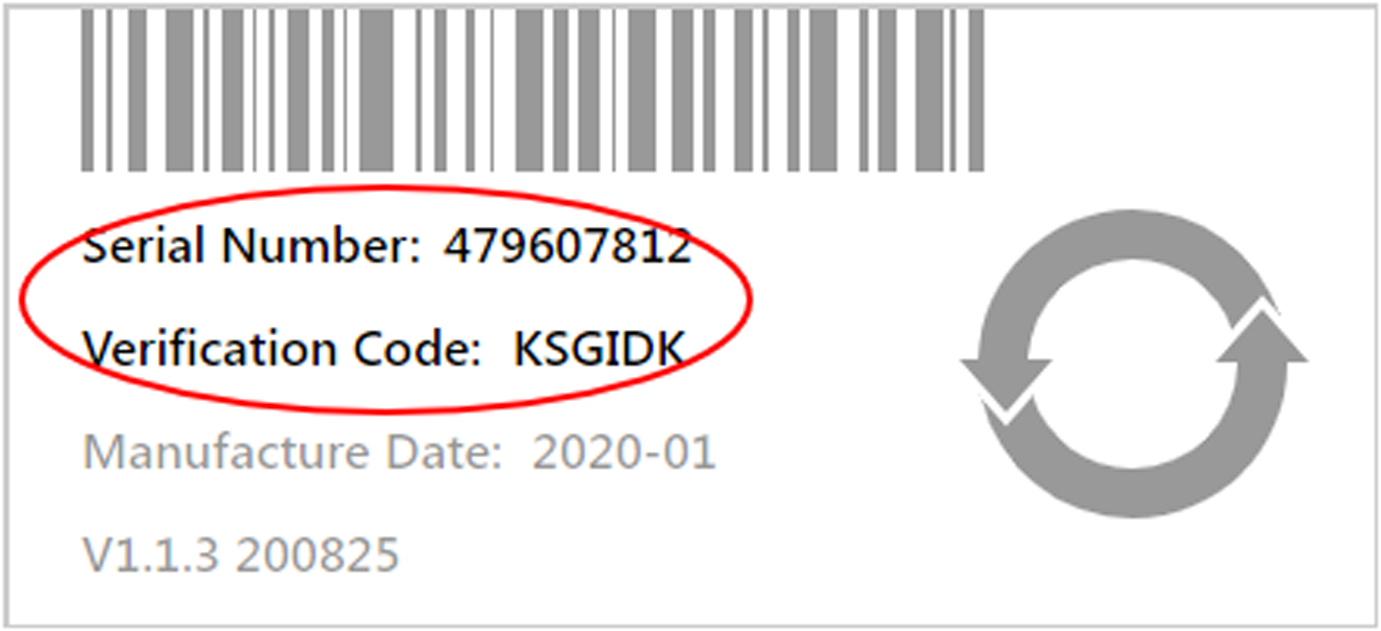
How to get the device serial number and verification code
Device Listing Number Format In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Download listing and proprietary name information through excel files that may be saved and searched locally. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. • manufacturer or specification developer must list device first; Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer may list • for combination products,. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Releasable establishment registration and listing information under the freedom of information act is available by.
From www.scribd.com
IEEE Device Numbers & Functions .pdf Relay Switch Device Listing Number Format • manufacturer or specification developer must list device first; Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Download listing and proprietary name information through excel files. Device Listing Number Format.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer may list • for combination products,. • manufacturer or specification developer must list device first; Governing issuance of medical. Device Listing Number Format.
From medicaldeviceacademy.com
FDA Registration and Listing for Medical Devices Device Listing Number Format Download listing and proprietary name information through excel files that may be saved and searched locally. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Releasable establishment registration and. Device Listing Number Format.
From www.resmed.com
Unique device identification ResMed Healthcare Professional Device Listing Number Format Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Releasable establishment registration and listing information under the freedom of information act is available by. • manufacturer or specification developer must list device first; In order to import your listed device into the united states, you’ll need to provide the registration number or. Device Listing Number Format.
From www.reghelps.com
Medical Device Registration & Device Listing Best FDA Specialist Device Listing Number Format Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import.. Device Listing Number Format.
From www.cariad.com.cn
Cariad medical announces the US FDA Approval! Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Then contract manufacturer or sterilizer may list • for combination products,. Download listing and proprietary name information through excel files that may be saved and searched locally. I have been working with our contract manufacturer to get their device listing updated. Device Listing Number Format.
From isgp.hik-proconnect.com
How to get the device serial number and verification code Device Listing Number Format Then contract manufacturer or sterilizer may list • for combination products,. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Releasable establishment registration and listing information under the freedom of information act is available by. (f) fda will assign one listing number for all devices exempt from. Device Listing Number Format.
From medicaldevicelicense.com
FDA Establishment Registration and Device Listing Device Listing Number Format Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Governing issuance of. Device Listing Number Format.
From bigfix-wiki.hcltechsw.com
Creating your own device list Blog Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of. Device Listing Number Format.
From www.reddit.com
Can't Find Medical Device Listing Number ANYWHERE r/dhl Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. • manufacturer or specification developer must list device first; I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Then contract manufacturer or sterilizer may list • for combination. Device Listing Number Format.
From www.hikvision.com
How to Check Device Serial Number FAQ Hikvision Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Then contract manufacturer or sterilizer may list • for combination products,. I have been working with our contract manufacturer to get their device listing. Device Listing Number Format.
From www.bharatagritech.com
The FDA Medical Device Classes Differences And Examples, 60 OFF Device Listing Number Format • manufacturer or specification developer must list device first; Then contract manufacturer or sterilizer may list • for combination products,. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can. Device Listing Number Format.
From blogs.3ds.com
FDA's Unique Device Identifier Successful Implementation Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. Then contract manufacturer or sterilizer may list • for combination products,. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Registration and listing provide the fda with the location of medical device establishments. Device Listing Number Format.
From www.ndsu.edu
Comparison of handheld 1lead ECG/EKG recorders Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. • manufacturer or specification developer must list device first; Download listing and proprietary name information through excel files that may be saved and searched. Device Listing Number Format.
From www.access.fda.gov
Create Listings for Medical Device Products Device Listing Number Format Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. Download listing and proprietary name information through excel files that may be saved and searched locally. Releasable establishment registration and listing information under the freedom of information act is available by. Then contract manufacturer or sterilizer may list • for. Device Listing Number Format.
From chinameddevice.com
CFDA New Medical Device Classification Catalog China Med Device Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Releasable establishment registration and listing information under the freedom of information act is available by. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. In order to import your listed device into the. Device Listing Number Format.
From medicaldeviceacademy.com
How to request FDA Device Classification 513(g) Alternative Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. Download listing and proprietary. Device Listing Number Format.
From www.access.preprod.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. • manufacturer or specification developer must list device first; I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Then contract manufacturer or sterilizer may list • for combination products,. (f) fda will. Device Listing Number Format.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Device Listing Number Format Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working. Device Listing Number Format.
From www.studocu.com
Standard device number Letters are sometimes added to specify the Device Listing Number Format I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Then contract manufacturer or sterilizer may list • for combination products,. Download listing and proprietary name information through excel files that may be saved and searched locally. (f) fda will assign one listing number for all devices exempt. Device Listing Number Format.
From www.hikvision.com
How to Check Device Serial Number FAQ Hikvision Device Listing Number Format I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Download listing and proprietary name information through excel files that may be saved and searched locally. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer. Device Listing Number Format.
From tecnomaquia.qualitypoolsboulder.com
How to Find the Serial Number of an iPhone, iPad, or iPod Touch Device Listing Number Format Download listing and proprietary name information through excel files that may be saved and searched locally. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. In order to. Device Listing Number Format.
From www.access.fda.gov
Annual Registration Device Listing Number Format Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. • manufacturer or specification developer must list device first; Then contract manufacturer or sterilizer may list • for combination products,. Registration. Device Listing Number Format.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Device Listing Number Format Then contract manufacturer or sterilizer may list • for combination products,. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. • manufacturer or specification developer must list device first; Download listing and proprietary name information through excel files that may be saved and searched locally. Releasable establishment registration and listing information under. Device Listing Number Format.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Device Listing Number Format I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. • manufacturer or specification developer must list device first; Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer may list • for combination products,. (f). Device Listing Number Format.
From www.hikvision.com
How to Check Device Serial Number FAQ Hikvision Device Listing Number Format Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed. Device Listing Number Format.
From www.chaithanya.com
How to Find Printer Model and Serial Numbers on Windows 11 Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer may list. Device Listing Number Format.
From techterms.com
Serial Number Definition Device Listing Number Format • manufacturer or specification developer must list device first; Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Then contract manufacturer or sterilizer may list • for combination products,. In order to import your listed device into the united states, you’ll need to provide the registration number or the owner/operation. Governing issuance. Device Listing Number Format.
From www.access.preprod.fda.gov
Device Registration and Listing Module (DRLM) StepbyStep Instructions Device Listing Number Format (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. Releasable establishment registration and listing information under the freedom of information act is available by. Download listing and proprietary name information through excel files that may be saved and searched locally. Governing issuance of medical device license listing and annual declaration,. Device Listing Number Format.
From www.access.fda.gov
Create Listings for Medical Device Products Device Listing Number Format Releasable establishment registration and listing information under the freedom of information act is available by. Download listing and proprietary name information through excel files that may be saved and searched locally. Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. In order to import your listed device into the. Device Listing Number Format.
From www.liststemplates.org
Device List Template Free List Templates Device Listing Number Format Then contract manufacturer or sterilizer may list • for combination products,. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Releasable establishment registration and listing information under the freedom of information act is available by. • manufacturer or specification developer must list device first; I have been working with our contract manufacturer. Device Listing Number Format.
From www.treedental.com
Trusted Dental Supplier Treedental Device Listing Number Format Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. (f) fda will assign one listing number for all devices exempt from premarket notification requirements under a single product. I have been working with our contract manufacturer to get their device listing updated for the fda so that we can. Device Listing Number Format.
From www.access.fda.gov
Create Listings for Medical Device Products Device Listing Number Format I have been working with our contract manufacturer to get their device listing updated for the fda so that we can import. • manufacturer or specification developer must list device first; Releasable establishment registration and listing information under the freedom of information act is available by. In order to import your listed device into the united states, you’ll need to. Device Listing Number Format.
From www.libertymanagement.us
FDA Registration Number FDA Registration Search Device Listing Number Format Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. • manufacturer or specification developer must list device first; Download listing and proprietary name information through excel files that may be saved and searched locally. In order to import your listed device into the united states, you’ll need to provide. Device Listing Number Format.
From www.access.preprod.fda.gov
Device Registration and Listing Module (DRLM) StepbyStep Instructions Device Listing Number Format Governing issuance of medical device license listing and annual declaration, manufacture and free sale certificate of the country of origin. Releasable establishment registration and listing information under the freedom of information act is available by. Download listing and proprietary name information through excel files that may be saved and searched locally. Registration and listing provide the fda with the location. Device Listing Number Format.