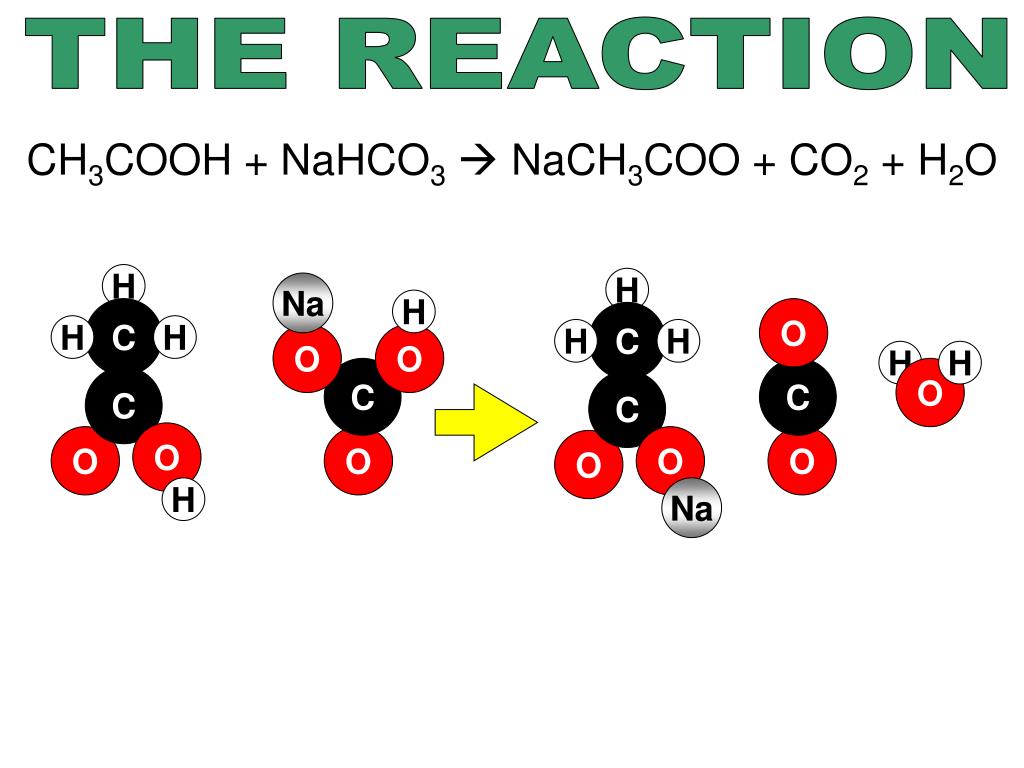
Limiting Reactant Lab Baking Soda And Vinegar . Circle your answer in columns 7 and. Therefore, as you add more baking soda the balloon gets bigger. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. One underlying assumption is that the baking soda is the only limiting reactant. Limiting reagent, vinegar or baking soda? Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. For this reaction, you need to. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. In in other words, there is essentially an unlimited supply of. In the first 2 flasks the limiting reagent is the baking soda. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar.
from www.slideserve.com
Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. One underlying assumption is that the baking soda is the only limiting reactant. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Therefore, as you add more baking soda the balloon gets bigger. For this reaction, you need to. In in other words, there is essentially an unlimited supply of. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Limiting reagent, vinegar or baking soda?
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint
Limiting Reactant Lab Baking Soda And Vinegar In in other words, there is essentially an unlimited supply of. Limiting reagent, vinegar or baking soda? If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. In in other words, there is essentially an unlimited supply of. In the first 2 flasks the limiting reagent is the baking soda. Circle your answer in columns 7 and. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Therefore, as you add more baking soda the balloon gets bigger. For this reaction, you need to. One underlying assumption is that the baking soda is the only limiting reactant. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons.
From studylib.net
Stoichiometry and Baking Soda Lab Limiting Reactant Lab Baking Soda And Vinegar If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. One underlying assumption is that the baking soda is the only limiting reactant. For this reaction, you need to. Using the data and your observations indicate which reactant the baking soda and. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Limiting reagent,. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. One underlying assumption is that the baking soda is the only limiting reactant. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. For this reaction, you need to. Using the data and your observations indicate which reactant the baking soda and vinegar are. Limiting Reactant Lab Baking Soda And Vinegar.
From www.studocu.com
6dutfigyihojpuiyliktdrjyhsteftyjgkuhj Baking soda & Vinegar Limiting Limiting Reactant Lab Baking Soda And Vinegar One underlying assumption is that the baking soda is the only limiting reactant. For this reaction, you need to. Limiting reagent, vinegar or baking soda? Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. In the first 2 flasks the limiting reagent is the baking soda. Using the data and your observations indicate which reactant the. Limiting Reactant Lab Baking Soda And Vinegar.
From www.studypool.com
SOLUTION Vinegar baking soda experiment Studypool Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. Therefore, as you add more baking soda the balloon gets bigger. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Limiting reagent, vinegar or baking soda? One underlying assumption. Limiting Reactant Lab Baking Soda And Vinegar.
From www.vecteezy.com
Baking Soda and Vinegar Balloon Science experiment, Chemistry Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. Limiting reagent, vinegar or baking soda? One underlying assumption is that the baking soda is the only limiting reactant. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. If the baking soda is the limiting. Limiting Reactant Lab Baking Soda And Vinegar.
From educationpossible.com
Baking Soda and Vinegar Balloon Experiment Education Possible Limiting Reactant Lab Baking Soda And Vinegar In in other words, there is essentially an unlimited supply of. Limiting reagent, vinegar or baking soda? In the first 2 flasks the limiting reagent is the baking soda. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. For this reaction, you need to. Therefore, as you add more baking soda. Limiting Reactant Lab Baking Soda And Vinegar.
From www.chegg.com
Solved Prelab Questions The reaction below occurs between Limiting Reactant Lab Baking Soda And Vinegar Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. For this reaction, you need to. Circle your answer in columns 7 and. In in other words, there is essentially an unlimited supply of. Using. Limiting Reactant Lab Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Casual Limiting Reagent Lab The Reaction Between Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. In the first 2 flasks the limiting reagent is the baking soda. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Using the data and your observations indicate which reactant the baking soda and. Limiting Reactant Lab Baking Soda And Vinegar.
From www.chegg.com
Solved Chem 1405 Lab 10 Stoichiometry and Limiting Reactant Limiting Reactant Lab Baking Soda And Vinegar Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. In in other words, there is essentially an unlimited supply of. Using the data and your observations indicate which reactant the baking soda and vinegar. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Circle your answer in columns 7 and. One underlying assumption is that the baking soda is the only. Limiting Reactant Lab Baking Soda And Vinegar.
From sydney-study-exams.blogspot.com
Stoichiometry Lab Vinegar And Baking Soda Answer Key 49+ Pages Solution Limiting Reactant Lab Baking Soda And Vinegar Circle your answer in columns 7 and. For this reaction, you need to. In in other words, there is essentially an unlimited supply of. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. One underlying assumption is that the baking soda is the only limiting reactant. In the first 2 flasks. Limiting Reactant Lab Baking Soda And Vinegar.
From shotprofessional22.gitlab.io
Ideal What Is The Balanced Chemical Equation For Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Circle your answer in columns 7 and. One underlying assumption is that the baking soda is the only limiting reactant. Therefore, as you add more baking soda the balloon gets bigger. Carbonic acid forms water and carbon dioxide, which causes the balloons. Limiting Reactant Lab Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Casual Limiting Reagent Lab The Reaction Between Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. In in other words, there is essentially an unlimited supply of. For this reaction, you need to. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Circle your. Limiting Reactant Lab Baking Soda And Vinegar.
From slideplayer.com
Limiting & Excess Reactants ppt download Limiting Reactant Lab Baking Soda And Vinegar One underlying assumption is that the baking soda is the only limiting reactant. In in other words, there is essentially an unlimited supply of. Therefore, as you add more baking soda the balloon gets bigger. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. If the baking soda is the limiting reactant than once all. Limiting Reactant Lab Baking Soda And Vinegar.
From www.pdffiller.com
Stoichiometry Baking Soda and Vinegar ReactionsStoichiometry (article Limiting Reactant Lab Baking Soda And Vinegar Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Circle your answer in columns 7 and. Limiting reagent, vinegar or baking soda? One underlying assumption is that the baking soda is the only limiting reactant. Vinegar and two different amounts of baking soda in plastic soda bottles. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar Limiting reagent, vinegar or baking soda? Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. In the first 2 flasks the limiting reagent is the baking soda. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Therefore, as you add more baking soda the balloon gets. Limiting Reactant Lab Baking Soda And Vinegar.
From ar.inspiredpencil.com
Vinegar And Baking Soda Balloon Experiment Data Limiting Reactant Lab Baking Soda And Vinegar Therefore, as you add more baking soda the balloon gets bigger. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda. Limiting Reactant Lab Baking Soda And Vinegar.
From worksheets.clipart-library.com
Baking Soda Stoichiometry Worksheet with Answers Limiting Reactant Lab Baking Soda And Vinegar If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar In in other words, there is essentially an unlimited supply of. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Vinegar and two different amounts of baking soda in. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Limiting reagent, vinegar or baking soda? For this reaction, you need to. Therefore, as you add more baking soda the balloon gets bigger. In the first 2 flasks the limiting reagent is the baking soda. Using the data and your observations indicate which reactant the baking soda. Limiting Reactant Lab Baking Soda And Vinegar.
From lessonlistelois.z13.web.core.windows.net
Limiting Reactant Lab Answers Limiting Reactant Lab Baking Soda And Vinegar Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. In the first 2 flasks the limiting reagent is the baking soda. Limiting reagent, vinegar or baking soda? Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. If the baking soda is the. Limiting Reactant Lab Baking Soda And Vinegar.
From www.studocu.com
Experiment Class Worksheet Baking Soda and Vinagar Experiment Limiting Reactant Lab Baking Soda And Vinegar Therefore, as you add more baking soda the balloon gets bigger. In the first 2 flasks the limiting reagent is the baking soda. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. One underlying assumption is that the baking soda is the only limiting reactant. Using the. Limiting Reactant Lab Baking Soda And Vinegar.
From www.numerade.com
SOLVED List each able to AND identify give a know complele that a Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. In in other words, there is essentially an unlimited supply of. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. For this reaction, you need to. One underlying assumption is that the baking soda is the only limiting reactant. Limiting reagent, vinegar or. Limiting Reactant Lab Baking Soda And Vinegar.
From pubs.acs.org
An Experimental Approach with a Twist Helping High School Students to Limiting Reactant Lab Baking Soda And Vinegar Limiting reagent, vinegar or baking soda? Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Carbonic acid forms water. Limiting Reactant Lab Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Casual Limiting Reagent Lab The Reaction Between Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. In the first 2 flasks the limiting reagent is the baking soda. In in other words, there is essentially an unlimited supply of. Limiting reagent, vinegar or baking soda? If the baking soda is the limiting reactant than once all the baking soda is used up the. Limiting Reactant Lab Baking Soda And Vinegar.
From exohykpht.blob.core.windows.net
Baking Soda And Vinegar Stoichiometry Lab at Warren Williams blog Limiting Reactant Lab Baking Soda And Vinegar Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. Therefore, as you add more baking soda the balloon gets bigger. Limiting reagent, vinegar or baking soda? If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. One underlying. Limiting Reactant Lab Baking Soda And Vinegar.
From www.vecteezy.com
Baking Soda and Vinegar Balloon Science experiment 21669329 Vector Art Limiting Reactant Lab Baking Soda And Vinegar Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. For this reaction, you need to. Circle your answer in columns 7 and. Therefore, as you add more baking soda the balloon gets bigger. Limiting reagent, vinegar or. Limiting Reactant Lab Baking Soda And Vinegar.
From www.alamy.com
Science experiment with baking soda and vinegar balloon illustration Limiting Reactant Lab Baking Soda And Vinegar Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. For this reaction, you need to. In the first 2 flasks the limiting reagent is the baking soda. Carbonic acid. Limiting Reactant Lab Baking Soda And Vinegar.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Limiting Reactant Lab Baking Soda And Vinegar Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. In in other words, there is essentially an unlimited supply of. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. Circle your answer in columns 7 and. Vinegar and. Limiting Reactant Lab Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Limiting Reactant Lab Baking Soda And Vinegar Limiting reagent, vinegar or baking soda? For this reaction, you need to. In the first 2 flasks the limiting reagent is the baking soda. Computing the mass of the limiting reactant (baking soda) in this lab, you will react baking soda with an excess of vinegar. Therefore, as you add more baking soda the balloon gets bigger. Circle your answer. Limiting Reactant Lab Baking Soda And Vinegar.
From slideplayer.com
Limiting & Excess Reactants ppt download Limiting Reactant Lab Baking Soda And Vinegar If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Vinegar and two different amounts of baking soda in plastic soda bottles with balloons. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial.. Limiting Reactant Lab Baking Soda And Vinegar.
From uwaterloo.ca
Sharing chemistry with the community Limiting and excess reagents Limiting Reactant Lab Baking Soda And Vinegar For this reaction, you need to. Therefore, as you add more baking soda the balloon gets bigger. Using the data and your observations indicate which reactant the baking soda and vinegar are for each trial. In the first 2 flasks the limiting reagent is the baking soda. Circle your answer in columns 7 and. Carbonic acid forms water and carbon. Limiting Reactant Lab Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Casual Limiting Reagent Lab The Reaction Between Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. Limiting reagent, vinegar or baking soda? In in other words, there is essentially an unlimited supply of. Circle your answer in columns 7 and. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. For this reaction, you need to. Therefore, as you add more. Limiting Reactant Lab Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Casual Limiting Reagent Lab The Reaction Between Vinegar And Baking Limiting Reactant Lab Baking Soda And Vinegar In the first 2 flasks the limiting reagent is the baking soda. If the baking soda is the limiting reactant than once all the baking soda is used up the reaction will stop and adding extra vinegar will have no. Carbonic acid forms water and carbon dioxide, which causes the balloons to inflate. One underlying assumption is that the baking. Limiting Reactant Lab Baking Soda And Vinegar.