Chlorine Dioxide Reaction With Hydrochloric Acid . Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. First, (equation 1), chlorine reacts with water to form. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The to main reactions are:
from igcsechemistryrevision.weebly.com
To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The to main reactions are: Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. First, (equation 1), chlorine reacts with water to form. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the.
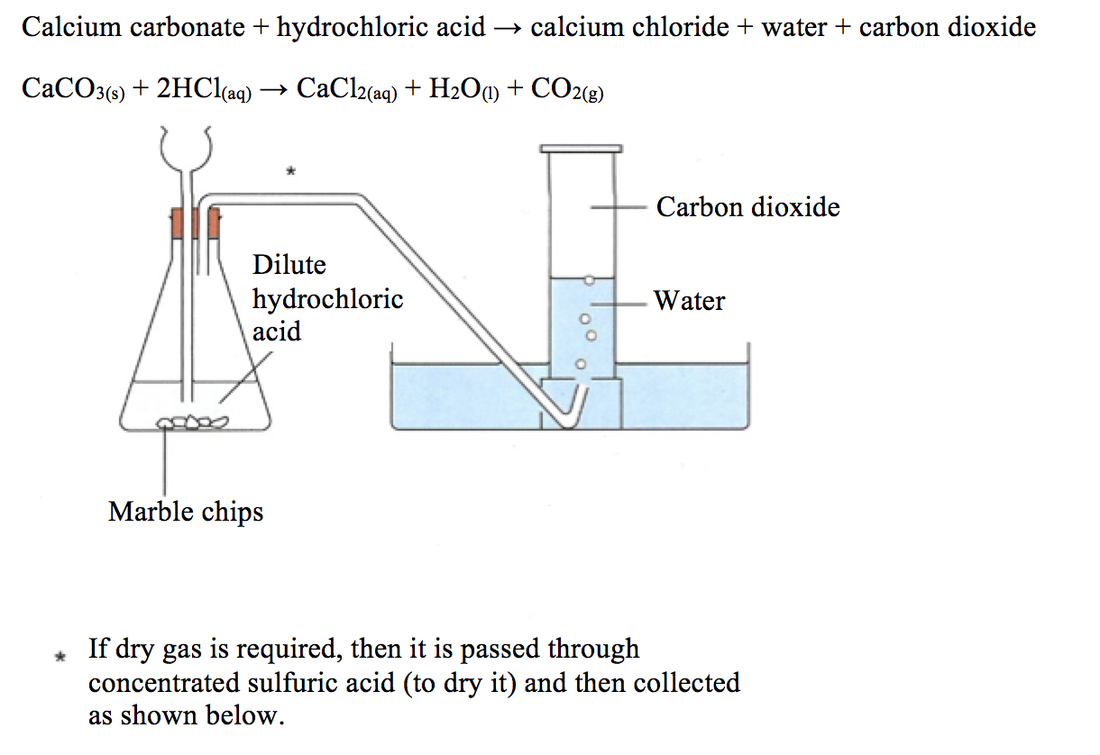
The Periodic Table iGCSE CHEMISTRY REVISION HELP
Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. The to main reactions are: Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. First, (equation 1), chlorine reacts with water to form. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The to main reactions are: Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Question 2 0.5pts 4HCl(aq) + MnO2(s) → MnCl2 + Cl2 Chlorine gas Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. First, (equation 1), chlorine reacts with water to form. The to main reactions are: 2naclo 2 + cl 2 ® 2clo 2 + 2nacl Sodium chlorate/hydrochloric acid. Chlorine Dioxide Reaction With Hydrochloric Acid.
From sportfishingf.blogspot.com
Manganese Dioxide Reacts With Hydrochloric Acid sportfishingf Chlorine Dioxide Reaction With Hydrochloric Acid 2naclo 2 + cl 2 ® 2clo 2 + 2nacl First, (equation 1), chlorine reacts with water to form. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The to main reactions are: To produce chlorine dioxide gas,. Chlorine Dioxide Reaction With Hydrochloric Acid.
From stock.adobe.com
Preparation of chlorine in laboratory. vector image illustration Chlorine Dioxide Reaction With Hydrochloric Acid The to main reactions are: To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Sodium chlorate/hydrochloric acid mixture has been. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.youtube.com
CaCO3 + HCl Calcium Carbonate + Hydrochloric Acid YouTube Chlorine Dioxide Reaction With Hydrochloric Acid First, (equation 1), chlorine reacts with water to form. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The to main reactions are: Chlorine dioxide is generally prepared using. Chlorine Dioxide Reaction With Hydrochloric Acid.
From cn.onnuri.org
calcium hydroxide and hydrochloric acid net ionic equation Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Chlorine Dioxide Reaction With Hydrochloric Acid To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. First, (equation 1), chlorine reacts with water to form. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT Lectures on sterilization and disinfection PowerPoint Chlorine Dioxide Reaction With Hydrochloric Acid First, (equation 1), chlorine reacts with water to form. The to main reactions are: The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Chlorine Dioxide Reaction With Hydrochloric Acid First, (equation 1), chlorine reacts with water to form. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The to main reactions are: Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The site of attack by. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT Water Treatment Disinfection Processes PowerPoint Presentation Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl Alternatively chlorine dioxide can be produced by reacting. Chlorine Dioxide Reaction With Hydrochloric Acid.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. Chlorine dioxide is generally prepared using. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.toppr.com
Chlorine is prepared in the laboratory by treating manganese dioxide Chlorine Dioxide Reaction With Hydrochloric Acid The to main reactions are: Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. The site of attack by. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Chlorine Dioxide Reaction With Hydrochloric Acid Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The to main reactions are: The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. First,. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Chlorine can be prepared in the laboratory by the reaction of Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.youtube.com
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O (calcium carbonate Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The present invention accordingly provides a method. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Chlorine is prepared in the laboratory bytreating manganese Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. First, (equation 1), chlorine reacts with water to form. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.scientific.net
of the Reaction for Generation of Chlorine Dioxide from Sodium Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. First, (equation 1), chlorine reacts with water to form. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Chlorine dioxide is generally. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous hydrochloric acid (HCl) reacts with aqueous chromium(VI Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. First, (equation 1), chlorine reacts with water to form. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Chlorine Dioxide Reaction With Hydrochloric Acid To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl First, (equation 1), chlorine reacts with water to form. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The to main reactions are: The site of. Chlorine Dioxide Reaction With Hydrochloric Acid.
From alexandrakruwanthony.blogspot.com
Sodium Thiosulphate and Hydrochloric Acid AlexandrakruwAnthony Chlorine Dioxide Reaction With Hydrochloric Acid The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. First, (equation 1), chlorine reacts with water to form. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. Alternatively chlorine dioxide can be produced by reacting the. Chlorine Dioxide Reaction With Hydrochloric Acid.
From stock.adobe.com
Vettoriale Stock Redox Reaction Infographic Diagram with example of Chlorine Dioxide Reaction With Hydrochloric Acid The to main reactions are: Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. First, (equation 1), chlorine reacts with water to form. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. First, (equation 1), chlorine reacts with water to form. The to main reactions are: 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The present invention. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Name of the Gas Produced When Magnesium Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.sarthaks.com
Why does chlorine & water forms when Magnese dioxide reacts with dilute Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The site of attack by chlorine is the carbon adjacent to. Chlorine Dioxide Reaction With Hydrochloric Acid.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chlorine Dioxide Reaction With Hydrochloric Acid Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. The site of attack by chlorine is. Chlorine Dioxide Reaction With Hydrochloric Acid.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Chlorine Dioxide Reaction With Hydrochloric Acid First, (equation 1), chlorine reacts with water to form. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The site of attack by. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
Chlorine forms from the reaction of hydrochloric acid with manganese(IV Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The to main reactions are: The present. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.researchgate.net
Energetically diagram of the pathways of the reaction chlorine dioxide Chlorine Dioxide Reaction With Hydrochloric Acid The to main reactions are: First, (equation 1), chlorine reacts with water to form. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.doubtnut.com
Chlorine is prepared in the laboratory by treating manganese dioxide Chlorine Dioxide Reaction With Hydrochloric Acid To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The to main reactions are: Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. 2naclo 2 + cl 2 ® 2clo 2. Chlorine Dioxide Reaction With Hydrochloric Acid.
From keplarllp.com
😀 Calcium hydrochloric acid. PSOW 04. 20190213 Chlorine Dioxide Reaction With Hydrochloric Acid 2naclo 2 + cl 2 ® 2clo 2 + 2nacl The to main reactions are: The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. First, (equation 1), chlorine reacts with water to form. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.toppr.com
Chlorine is prepared in the laboratory by treating manganese dioxide Chlorine Dioxide Reaction With Hydrochloric Acid Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The present invention accordingly provides a method of preparing chlorine dioxide from hydrochloric acid and sodium chlorite in the. Sodium. Chlorine Dioxide Reaction With Hydrochloric Acid.
From brainly.in
Chlorine is prepared in the laboratory by treating manganese dioxide Chlorine Dioxide Reaction With Hydrochloric Acid To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. Alternatively chlorine dioxide can be produced by reacting the sodium chlorite with hydrochloric acid. The to main reactions are: Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. Sodium chlorate/hydrochloric acid mixture has been actively used. Chlorine Dioxide Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. 2naclo 2 + cl 2 ® 2clo 2 + 2nacl First, (equation 1), chlorine reacts with water to form. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. To. Chlorine Dioxide Reaction With Hydrochloric Acid.
From cleanhospital.com
Chlorine Dioxide Chlorine Dioxide Reaction With Hydrochloric Acid Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. Sodium chlorate/hydrochloric acid mixture has been actively used as chlorinating agent and wikipedia did mention that it. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. The to main. Chlorine Dioxide Reaction With Hydrochloric Acid.
From fado.vn
Mua CrystalClearLab Chlorine Dioxide Hydrochloric Acid and Sodium Chlorine Dioxide Reaction With Hydrochloric Acid To produce chlorine dioxide gas, hydrochloric acid (hcl) or chlorine is brought together with sodium chlorite. The site of attack by chlorine is the carbon adjacent to the one bearing oxygen, and this attack, wherein the hydrogen atoms are. Chlorine dioxide is generally prepared using sodium chlorite or sodium chlorate by reduction reaction in acidic conditions. Alternatively chlorine dioxide can. Chlorine Dioxide Reaction With Hydrochloric Acid.