Bromine Atom Gains Electron . Atoms of group 16 gain two electrons and form ions with a 2− charge,. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet.
from www.alamy.com
Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms can gain or lose electrons to become ions.
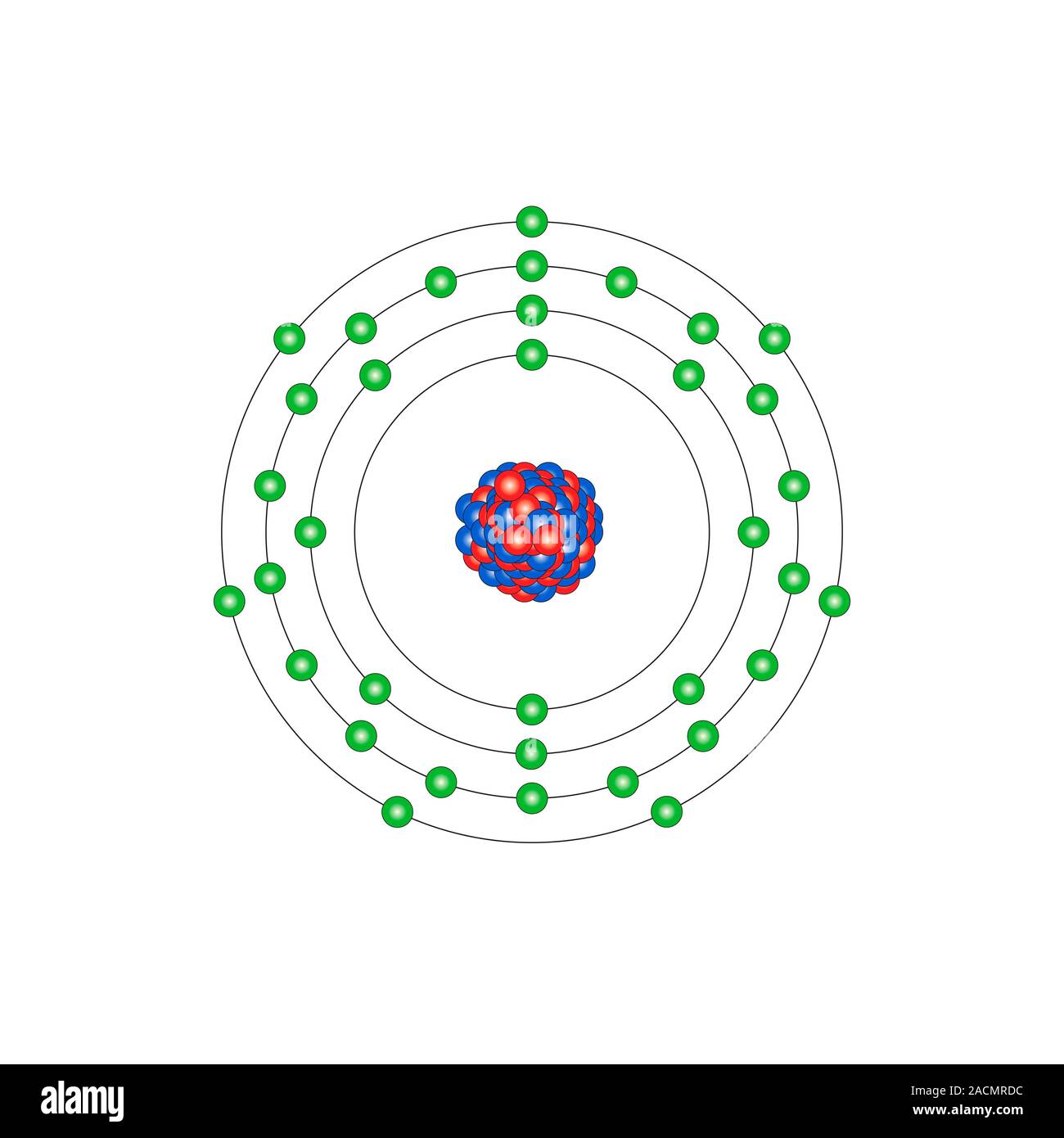
Bromine (Br). Diagram of the nuclear composition and electron
Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2−. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure Stock Vector (Royalty Free Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 17 gain one electron and form anions with a 1− charge; Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms can gain or lose electrons to become ions.. Bromine Atom Gains Electron.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions. Some atoms have nearly eight electrons in their valence shell and can gain. Bromine Atom Gains Electron.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. Atoms can gain or lose electrons to become ions. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Some atoms have nearly eight electrons in their valence shell and. Bromine Atom Gains Electron.
From www.vectorstock.com
Symbol and electron diagram for Bromine Royalty Free Vector Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. When an atom loses an electron it gains. Bromine Atom Gains Electron.
From mavink.com
Aufbau Diagram For Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2− charge,. When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and. Bromine Atom Gains Electron.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an. Bromine Atom Gains Electron.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and form. Bromine Atom Gains Electron.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for. Bromine Atom Gains Electron.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Some atoms have nearly eight electrons in their. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions. Bromine Atom Gains Electron.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. Some atoms have. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Some atoms have nearly eight electrons in. Bromine Atom Gains Electron.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Atom Gains Electron Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. Atoms can gain or lose electrons to become ions.. Bromine Atom Gains Electron.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Bromine Atom Gains Electron Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms can gain or lose electrons to become ions. When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when. Bromine Atom Gains Electron.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and form ions with. Bromine Atom Gains Electron.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions.. Bromine Atom Gains Electron.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure Stock Vector (Royalty Free Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and form ions with a 2−.. Bromine Atom Gains Electron.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and form. Bromine Atom Gains Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 16 gain two electrons and. Bromine Atom Gains Electron.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a. Bromine Atom Gains Electron.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Atom Gains Electron Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. When an. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and form ions with a 2− charge,. When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase,. Bromine Atom Gains Electron.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to. Bromine Atom Gains Electron.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Atoms can gain or lose electrons to become ions. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. When an atom. Bromine Atom Gains Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Gains Electron Atoms can gain or lose electrons to become ions. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 16 gain two electrons and form ions with. Bromine Atom Gains Electron.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Gains Electron Atoms can gain or lose electrons to become ions. Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 16 gain two electrons and form ions with a 2−. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When an. Bromine Atom Gains Electron.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. Atoms of group 17 gain one electron and form anions with a 1− charge; When an atom loses an electron it gains a positive charge and is called a cation. An atom of bromine in the gas phase, for example, gives off energy when it gains an. Bromine Atom Gains Electron.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Atom Gains Electron Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 16 gain two electrons and form ions with a 2−. An atom of bromine in the gas phase, for example,. Bromine Atom Gains Electron.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Atom Gains Electron Atoms of group 17 gain one electron and form anions with a 1− charge; An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and. Bromine Atom Gains Electron.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Gains Electron Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with. Bromine Atom Gains Electron.
From stewart-switch.com
Electron Dot Diagram For Bromine Bromine Atom Gains Electron When an atom loses an electron it gains a positive charge and is called a cation. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and. Bromine Atom Gains Electron.
From www.istockphoto.com
Bromine Atom Electron Shell Stock Illustration Download Image Now Bromine Atom Gains Electron Atoms of group 16 gain two electrons and form ions with a 2−. Atoms can gain or lose electrons to become ions. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When an atom. Bromine Atom Gains Electron.