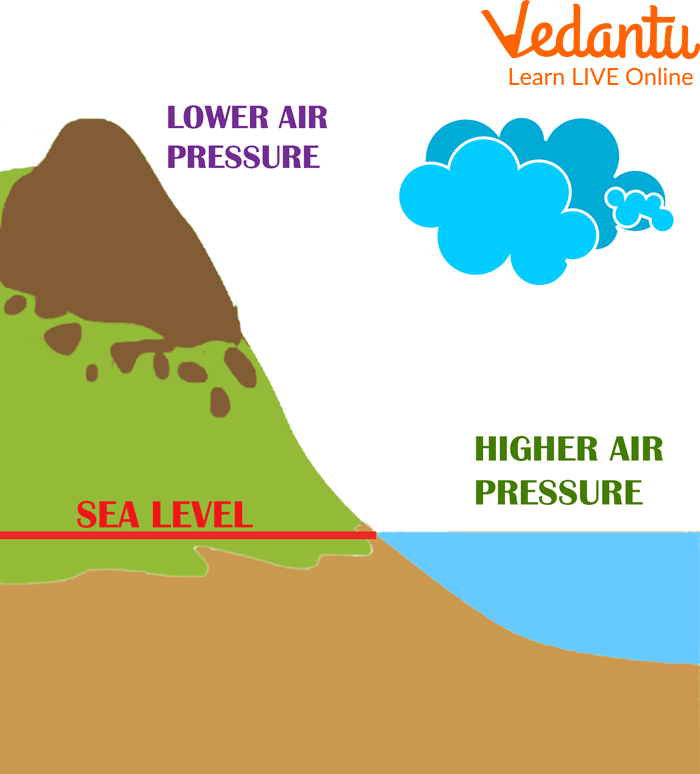
What Does Evaporation Do To Atmospheric Pressure . As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium is reached where an equal. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. [hide solution] decreased the atmospheric. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop?
from www.vedantu.com
[hide solution] decreased the atmospheric. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If the container is closed, an equilibrium is reached where an equal. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or.
Air Pressure on Water Learn Definition, Facts & Uses
What Does Evaporation Do To Atmospheric Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? [hide solution] decreased the atmospheric. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. If the container is closed, an equilibrium is reached where an equal.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa. What Does Evaporation Do To Atmospheric Pressure.
From slideplayer.com
LEARNING TARGET WATER CYCLE What are the atmospheric conditions needed What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the liquid is in an open container. What Does Evaporation Do To Atmospheric Pressure.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. [hide solution] decreased the atmospheric. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. How. What Does Evaporation Do To Atmospheric Pressure.
From saylordotorg.github.io
Vapor Pressure What Does Evaporation Do To Atmospheric Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium. What Does Evaporation Do To Atmospheric Pressure.
From invaderxan.tumblr.com
Mostly Harmless — Phase diagram for water. The triple point is the... What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an. What Does Evaporation Do To Atmospheric Pressure.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium is reached where an equal. [hide solution] decreased the atmospheric. How does the lowered atmospheric pressure speed the drying process, and why does. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium is reached where an equal. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when. What Does Evaporation Do To Atmospheric Pressure.
From www.slideshare.net
Evaporation ( simple ppt ) What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. If the container is closed, an equilibrium is reached where an equal. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. If the container is closed, an equilibrium is reached where an equal. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Part AUnit 2 PowerPoint Presentation, free download ID2484640 What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the container is closed, an equilibrium is reached where an equal. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or. What Does Evaporation Do To Atmospheric Pressure.
From www.pinterest.com
Evaporation Easy Science Science flashcards, Evaporation, Physics What Does Evaporation Do To Atmospheric Pressure If the container is closed, an equilibrium is reached where an equal. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the. What Does Evaporation Do To Atmospheric Pressure.
From dustinajohnsono.blob.core.windows.net
Evaporation And Condensation Are Both Types Of Vaporization at What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. [hide solution] decreased the atmospheric. If the. What Does Evaporation Do To Atmospheric Pressure.
From shaunmwilliams.com
Chapter 10 Presentation What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa. What Does Evaporation Do To Atmospheric Pressure.
From youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium is reached where an equal. [hide solution] decreased the atmospheric. How does the lowered atmospheric pressure speed the drying process, and why does. What Does Evaporation Do To Atmospheric Pressure.
From philschatz.com
Gauge Pressure, Absolute Pressure, and Pressure Measurement · Physics What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the container is closed, an equilibrium is reached where an equal. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when. What Does Evaporation Do To Atmospheric Pressure.
From socratic.org
How does atmospheric pressure change with altitude? Socratic What Does Evaporation Do To Atmospheric Pressure If the container is closed, an equilibrium is reached where an equal. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed. What Does Evaporation Do To Atmospheric Pressure.
From www.vrogue.co
Vapor Pressure And Evaporation In Vacuum Furnaces vrogue.co What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? [hide solution] decreased the atmospheric. If the container. What Does Evaporation Do To Atmospheric Pressure.
From saltworkconsultants.com
Physics of evaporation What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. [hide solution] decreased the atmospheric. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the container. What Does Evaporation Do To Atmospheric Pressure.
From www.pinterest.com
Atmosphere characteristics, air pressure Molecule diagram, Earth and What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? Vapor pressure or equilibrium vapor pressure is. What Does Evaporation Do To Atmospheric Pressure.
From www.vedantu.com
Air Pressure on Water Learn Definition, Facts & Uses What Does Evaporation Do To Atmospheric Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. [hide solution] decreased the atmospheric. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the. What Does Evaporation Do To Atmospheric Pressure.
From shinestarpk.blogspot.com
Education World What Is The Evaporation? What Does Evaporation Do To Atmospheric Pressure How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the liquid is in an open container. What Does Evaporation Do To Atmospheric Pressure.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. How does. What Does Evaporation Do To Atmospheric Pressure.
From byjus.com
The instrument used for measuring air pressure is called What Does Evaporation Do To Atmospheric Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. If the liquid is in an open. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Does Evaporation Do To Atmospheric Pressure How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the container is closed, an equilibrium is reached where an equal. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the. What Does Evaporation Do To Atmospheric Pressure.
From sciencenotes.org
Evaporative Cooler How It Works and Examples What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. If the container is closed, an equilibrium is reached where an equal. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature. What Does Evaporation Do To Atmospheric Pressure.
From slideplayer.com
EVAPORATION Definition Process by which water is changed from the What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the liquid is in an open container. What Does Evaporation Do To Atmospheric Pressure.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? What Does Evaporation Do To Atmospheric Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. [hide solution] decreased the atmospheric. If the. What Does Evaporation Do To Atmospheric Pressure.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Does Evaporation Do To Atmospheric Pressure How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. If the container is closed, an equilibrium. What Does Evaporation Do To Atmospheric Pressure.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Does Evaporation Do To Atmospheric Pressure How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the container is closed, an equilibrium is reached where an equal. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1. What Does Evaporation Do To Atmospheric Pressure.
From www.malteseislandsweather.com
Note 5 Atmospheric Pressure Maltese Islands Weather What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. If the container is closed, an equilibrium is reached where an equal. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a. What Does Evaporation Do To Atmospheric Pressure.
From www.slideserve.com
PPT Atmospheric Pressure PowerPoint Presentation, free download ID What Does Evaporation Do To Atmospheric Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. How does the lowered atmospheric pressure. What Does Evaporation Do To Atmospheric Pressure.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help What Does Evaporation Do To Atmospheric Pressure If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. As the temperature of a liquid. What Does Evaporation Do To Atmospheric Pressure.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapour pressure becomes equal to 1 atmosphere (or 101325 pa or. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? As the. What Does Evaporation Do To Atmospheric Pressure.
From www.vrogue.co
11 Examples Of Evaporation In Our Daily Life Explaine vrogue.co What Does Evaporation Do To Atmospheric Pressure [hide solution] decreased the atmospheric. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. How does the lowered atmospheric pressure speed the drying process, and why does it cause the temperature of the food to drop? If the liquid. What Does Evaporation Do To Atmospheric Pressure.