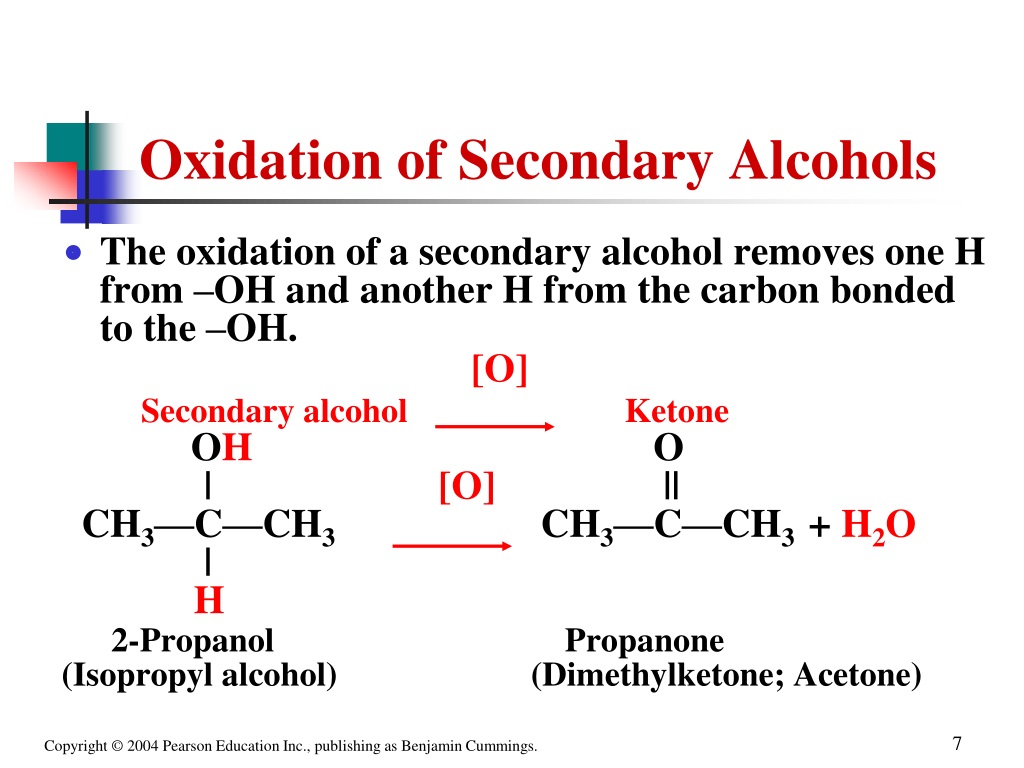
Are Phenols Secondary Alcohols . Hydroxyl groups attached to aromatic rings are called, “phenols”. Phenols are about a million times more acidic than alcohols (table 17.1). They react with aqueous sodium hydroxide (naoh) to form salts. Explain, in terms of inductive and resonance effects, why a given substituted. Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Phenols differ from alcohols in that they are slightly acidic in water. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Note that not all functional groups containing oh are alcohols. If the oh is attached to a carbonyl (c=o), that. Secondary alcohols can only oxidize once, which results in ketones.
from www.slideserve.com
Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. If the oh is attached to a carbonyl (c=o), that. Phenols are about a million times more acidic than alcohols (table 17.1). Note that not all functional groups containing oh are alcohols. Phenols differ from alcohols in that they are slightly acidic in water. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Secondary alcohols can only oxidize once, which results in ketones. Hydroxyl groups attached to aromatic rings are called, “phenols”. Explain why phenols are more acidic than alcohols. They react with aqueous sodium hydroxide (naoh) to form salts.
PPT Chapter 14 Alcohols, Phenols, Ethers, and Thiols PowerPoint
Are Phenols Secondary Alcohols Hydroxyl groups attached to aromatic rings are called, “phenols”. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Explain why phenols are more acidic than alcohols. Phenols differ from alcohols in that they are slightly acidic in water. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. They react with aqueous sodium hydroxide (naoh) to form salts. If the oh is attached to a carbonyl (c=o), that. Hydroxyl groups attached to aromatic rings are called, “phenols”. Note that not all functional groups containing oh are alcohols. Secondary alcohols can only oxidize once, which results in ketones. Phenols are about a million times more acidic than alcohols (table 17.1). Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Explain, in terms of inductive and resonance effects, why a given substituted.
From www.askiitians.com
Nomenclature of Alcohols Phenols and Ethers Study Material for IIT Are Phenols Secondary Alcohols Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Phenols are about a million times more acidic than alcohols (table 17.1). They are therefore soluble in dilute aqueous naoh and can often. Are Phenols Secondary Alcohols.
From study.com
Alcohols & Alkanols Classification & Functional Group Video & Lesson Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Note that not all functional groups containing oh are alcohols. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. They are therefore. Are Phenols Secondary Alcohols.
From www.academia.edu
(DOC) Experiment 12 Identification of Alcohols and Phenols nor misbah Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Hydroxyl groups attached to aromatic rings are called, “phenols”. Note that not all functional groups containing oh are alcohols. Phenols are about a million times more acidic than alcohols (table 17.1). Explain, in terms of inductive and resonance effects,. Are Phenols Secondary Alcohols.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID6508687 Are Phenols Secondary Alcohols If the oh is attached to a carbonyl (c=o), that. Phenols differ from alcohols in that they are slightly acidic in water. Phenols are about a million times more acidic than alcohols (table 17.1). Note that not all functional groups containing oh are alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture. Are Phenols Secondary Alcohols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Are Phenols Secondary Alcohols Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Note that not all functional groups containing oh are alcohols. Explain why phenols are more acidic than alcohols. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Hydroxyl groups attached to aromatic. Are Phenols Secondary Alcohols.
From slideplayer.com
Alcohols and Phenols by SHITTU T. O ppt download Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenols differ from alcohols in that they are slightly acidic in water. Explain why phenols are more acidic than alcohols. Alcohols are classified as primary (1°), secondary (2°), or. Are Phenols Secondary Alcohols.
From www.meritnation.com
Give two reason why phenols are more stronger acids than alcohols Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. If the oh is attached to a carbonyl (c=o), that. They react with aqueous sodium hydroxide (naoh) to form salts. Explain, in terms of inductive and resonance effects, why a given substituted. Tertiary alcohols cannot be oxidized, as the. Are Phenols Secondary Alcohols.
From slideplayer.com
Alcohols and Phenols ppt download Are Phenols Secondary Alcohols Hydroxyl groups attached to aromatic rings are called, “phenols”. Phenols differ from alcohols in that they are slightly acidic in water. Note that not all functional groups containing oh are alcohols. Phenols are about a million times more acidic than alcohols (table 17.1). Explain, in terms of inductive and resonance effects, why a given substituted. If the oh is attached. Are Phenols Secondary Alcohols.
From askfilo.com
CHEMICAL REACTIONS OF ALCOHOLS AND PHENOLS Alcohols are versatile compoun.. Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain, in terms of inductive and resonance effects, why a given substituted. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Hydroxyl groups attached to aromatic rings. Are Phenols Secondary Alcohols.
From www.studocu.com
6 Alcohols and Phenols lab report Experiment 6 Alcohols and Are Phenols Secondary Alcohols Phenols differ from alcohols in that they are slightly acidic in water. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. They react with aqueous sodium hydroxide (naoh) to form salts. Note that not all functional groups containing oh are alcohols. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals. Are Phenols Secondary Alcohols.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download Are Phenols Secondary Alcohols Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Secondary alcohols can only oxidize once, which results in ketones. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given substituted. Hydroxyl groups attached to aromatic rings are called, “phenols”. They are therefore soluble in. Are Phenols Secondary Alcohols.
From saylordotorg.github.io
Alcohols Nomenclature and Classification Are Phenols Secondary Alcohols Phenols differ from alcohols in that they are slightly acidic in water. If the oh is attached to a carbonyl (c=o), that. Secondary alcohols can only oxidize once, which results in ketones. Note that not all functional groups containing oh are alcohols. Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can. Are Phenols Secondary Alcohols.
From studylib.net
Alcohols, Phenols and Ethers Are Phenols Secondary Alcohols Hydroxyl groups attached to aromatic rings are called, “phenols”. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenols are about a million times more acidic than alcohols (table 17.1). Note that not all functional groups containing oh are alcohols. Explain why phenols are more acidic than alcohols.. Are Phenols Secondary Alcohols.
From sciencebyjus.blogspot.com
Acidic Nature of Phenols? Why phenols are more acidic than alcohol Are Phenols Secondary Alcohols If the oh is attached to a carbonyl (c=o), that. Secondary alcohols can only oxidize once, which results in ketones. Phenols are about a million times more acidic than alcohols (table 17.1). Explain why phenols are more acidic than alcohols. Hydroxyl groups attached to aromatic rings are called, “phenols”. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°),. Are Phenols Secondary Alcohols.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Note that not all functional groups containing oh are alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Explain why phenols. Are Phenols Secondary Alcohols.
From www.numerade.com
SOLVEDAlcohols/Phenols/Thiols 1. Draw the functional groups that are Are Phenols Secondary Alcohols Secondary alcohols can only oxidize once, which results in ketones. Note that not all functional groups containing oh are alcohols. They react with aqueous sodium hydroxide (naoh) to form salts. Phenols are about a million times more acidic than alcohols (table 17.1). Hydroxyl groups attached to aromatic rings are called, “phenols”. Explain why phenols are more acidic than alcohols. They. Are Phenols Secondary Alcohols.
From readchemistry.com
Structure and Classification of Alcohols Read Chemistry Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Phenols differ from alcohols in that they are slightly acidic in water. Secondary alcohols can only oxidize once, which results in ketones. Explain. Are Phenols Secondary Alcohols.
From slideplayer.com
Alcohols and Phenols ppt download Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Note that not all functional groups containing oh are alcohols. Explain why phenols are more acidic than alcohols. Hydroxyl groups attached to aromatic rings are called, “phenols”. Phenols differ from alcohols in that they. Are Phenols Secondary Alcohols.
From www.vrogue.co
Types Of Alcohols Primary Secondary Tertiary Alcohols vrogue.co Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Explain, in terms of inductive and resonance effects, why a given substituted. Explain why phenols are more acidic than alcohols. Note that not all functional groups containing oh are alcohols. Phenols are about a million times more acidic than alcohols (table 17.1). Phenols differ from alcohols in that they are. Are Phenols Secondary Alcohols.
From www.samareducation.com
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11 Are Phenols Secondary Alcohols If the oh is attached to a carbonyl (c=o), that. Secondary alcohols can only oxidize once, which results in ketones. Explain why phenols are more acidic than alcohols. Hydroxyl groups attached to aromatic rings are called, “phenols”. They react with aqueous sodium hydroxide (naoh) to form salts. Phenols are about a million times more acidic than alcohols (table 17.1). Note. Are Phenols Secondary Alcohols.
From www.masternotes.in
Alcohols phenols and ethers class 12 notes Master Notes Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Secondary alcohols can only oxidize once, which results in ketones. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. If the oh is attached to a carbonyl. Are Phenols Secondary Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Are Phenols Secondary Alcohols They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Hydroxyl groups attached to aromatic rings are called, “phenols”. Note that not all functional groups containing oh are alcohols. Explain why phenols are more acidic than alcohols. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending. Are Phenols Secondary Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Are Phenols Secondary Alcohols Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Phenols are about a million times more acidic than alcohols (table 17.1). Secondary alcohols can only oxidize once, which results in ketones. Phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium. Are Phenols Secondary Alcohols.
From chem.libretexts.org
1.6 Functional Groups Chemistry LibreTexts Are Phenols Secondary Alcohols Explain why phenols are more acidic than alcohols. Note that not all functional groups containing oh are alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen.. Are Phenols Secondary Alcohols.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download Are Phenols Secondary Alcohols Hydroxyl groups attached to aromatic rings are called, “phenols”. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. They react with aqueous sodium hydroxide (naoh) to form salts. Phenols are about a million times more acidic than alcohols (table 17.1). Secondary alcohols can only oxidize once, which results. Are Phenols Secondary Alcohols.
From www.geeksforgeeks.org
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Explain why phenols are more acidic than alcohols. Secondary alcohols can only oxidize once, which results in ketones. Phenols differ from alcohols in that they are slightly acidic in water. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the. Are Phenols Secondary Alcohols.
From slidetodoc.com
ALCOHOLS PHENOLS and ETHERS Functional group of an Are Phenols Secondary Alcohols Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Note that not all functional groups containing oh are alcohols. Phenols differ from alcohols in that they are slightly acidic in water. If the oh is attached to a carbonyl (c=o), that. Explain why phenols are more acidic than. Are Phenols Secondary Alcohols.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Are Phenols Secondary Alcohols Explain, in terms of inductive and resonance effects, why a given substituted. Hydroxyl groups attached to aromatic rings are called, “phenols”. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Note that not all functional groups containing oh are alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated. Are Phenols Secondary Alcohols.
From www.slideserve.com
PPT Chapter 14 Alcohols, Phenols, Ethers, and Thiols PowerPoint Are Phenols Secondary Alcohols Phenols differ from alcohols in that they are slightly acidic in water. If the oh is attached to a carbonyl (c=o), that. Note that not all functional groups containing oh are alcohols. Phenols are about a million times more acidic than alcohols (table 17.1). They react with aqueous sodium hydroxide (naoh) to form salts. Tertiary alcohols cannot be oxidized, as. Are Phenols Secondary Alcohols.
From www.doubtnut.com
Carboxylic acids are more acidic than phenols and alchols. Explin Are Phenols Secondary Alcohols Explain, in terms of inductive and resonance effects, why a given substituted. They react with aqueous sodium hydroxide (naoh) to form salts. Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. Secondary alcohols can only oxidize once, which results in ketones. If the oh is attached to a. Are Phenols Secondary Alcohols.
From studylib.net
Alcohols and Phenols Are Phenols Secondary Alcohols If the oh is attached to a carbonyl (c=o), that. Explain why phenols are more acidic than alcohols. Hydroxyl groups attached to aromatic rings are called, “phenols”. They react with aqueous sodium hydroxide (naoh) to form salts. Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of. Phenols differ from alcohols in that they. Are Phenols Secondary Alcohols.
From www.embibe.com
Alcohols, Phenols and Ethers Embibe Exams Are Phenols Secondary Alcohols They react with aqueous sodium hydroxide (naoh) to form salts. Phenols are about a million times more acidic than alcohols (table 17.1). Hydroxyl groups attached to aromatic rings are called, “phenols”. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given substituted. Alcohols are classified as primary (1°), secondary (2°), or. Are Phenols Secondary Alcohols.
From www.vedantu.com
JEE Advanced 2023 Revision Notes for Alcohols, Phenols and Ethers Are Phenols Secondary Alcohols Explain, in terms of inductive and resonance effects, why a given substituted. They react with aqueous sodium hydroxide (naoh) to form salts. Secondary alcohols can only oxidize once, which results in ketones. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Tertiary alcohols cannot be oxidized, as the. Are Phenols Secondary Alcohols.
From www.cleariitmedical.com
Alcohols, Phenols and Ethers Chemistry Notes for IITJEE/NEET Are Phenols Secondary Alcohols If the oh is attached to a carbonyl (c=o), that. Explain why phenols are more acidic than alcohols. Secondary alcohols can only oxidize once, which results in ketones. Phenols are about a million times more acidic than alcohols (table 17.1). Explain, in terms of inductive and resonance effects, why a given substituted. Note that not all functional groups containing oh. Are Phenols Secondary Alcohols.
From www.chegg.com
Solved Alcohols and Phenols Goals Determine chemical and Are Phenols Secondary Alcohols Tertiary alcohols cannot be oxidized, as the carbon atom has no orbitals left for forming a double bond with the oxygen. If the oh is attached to a carbonyl (c=o), that. Note that not all functional groups containing oh are alcohols. Secondary alcohols can only oxidize once, which results in ketones. Explain why phenols are more acidic than alcohols. Phenols. Are Phenols Secondary Alcohols.