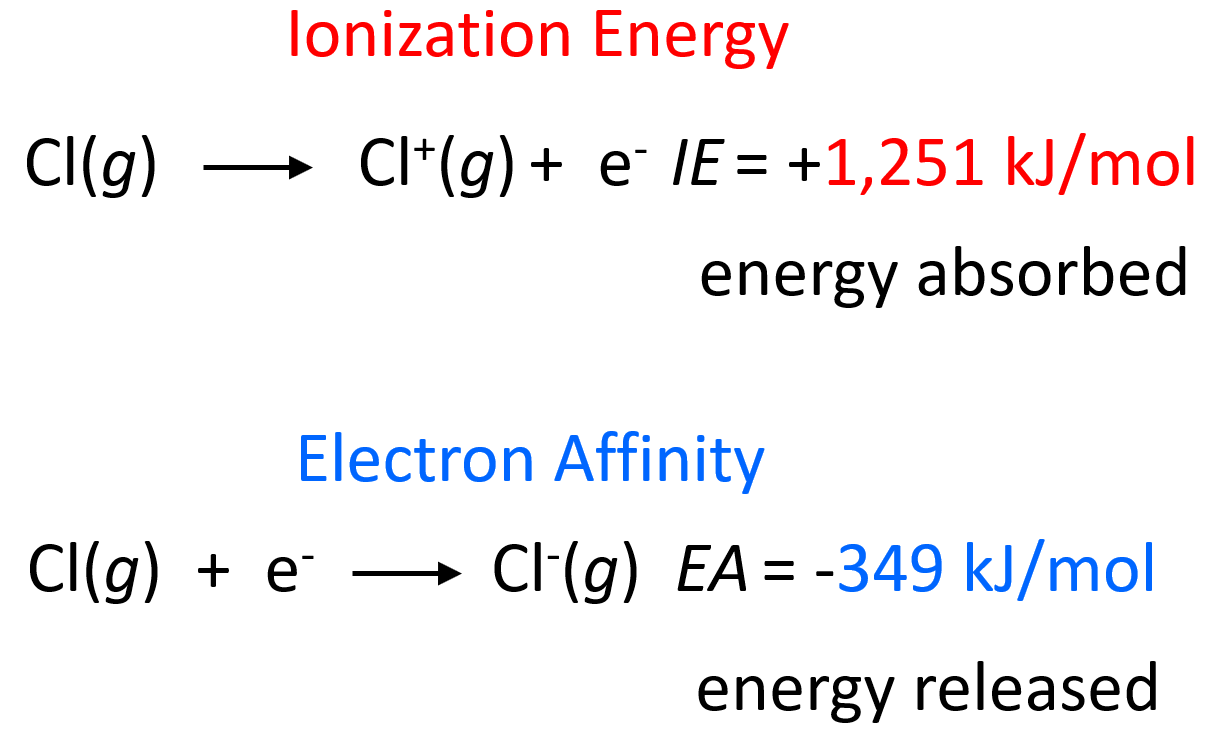
Electron Affinity Value Of Chlorine . The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. 103 rows electron affinity is related to electronegativity of elements. By convention, the negative sign shows a release of energy. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Values in parentheses ( ) are predicted values. 119 rows periodic table with electron affinity values is shown above. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. Unlike electronegativity, electron affinity is a. As the name suggests, electron affinity is the ability of an atom to accept an electron. The values of electron affinity are given in kj/mol. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Electron affinity of chlorine is 349 kj/mol. Simply speaking, the greater the affinity of electrons, the more. The greater the attractive forces between the.
from general.chemistrysteps.com
Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. Unlike electronegativity, electron affinity is a. The values of electron affinity are given in kj/mol. 103 rows electron affinity is related to electronegativity of elements. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. By convention, the negative sign shows a release of energy. Electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is. As the name suggests, electron affinity is the ability of an atom to accept an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom.
Electron Affinity Chemistry Steps
Electron Affinity Value Of Chlorine The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. The greater the attractive forces between the. 119 rows periodic table with electron affinity values is shown above. The values of electron affinity are given in kj/mol. Values in parentheses ( ) are predicted values. 103 rows electron affinity is related to electronegativity of elements. Electron affinity of chlorine is 349 kj/mol. Unlike electronegativity, electron affinity is a. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. As the name suggests, electron affinity is the ability of an atom to accept an electron. By convention, the negative sign shows a release of energy. Simply speaking, the greater the affinity of electrons, the more.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Value Of Chlorine Electron affinity of chlorine is 349 kj/mol. 119 rows periodic table with electron affinity values is shown above. Values in parentheses ( ) are predicted values. The values of electron affinity are given in kj/mol. Unlike electronegativity, electron affinity is a. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released. Electron Affinity Value Of Chlorine.
From www.nuclear-power.com
Nitrogen Electron Affinity Electronegativity Ionization Energy of Electron Affinity Value Of Chlorine Values in parentheses ( ) are predicted values. The values of electron affinity are given in kj/mol. 103 rows electron affinity is related to electronegativity of elements. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Unlike electronegativity, electron affinity is a. Electron affinity of chlorine is 349 kj/mol. As the name suggests, electron affinity. Electron Affinity Value Of Chlorine.
From mavink.com
Element Electronegativity Chart Electron Affinity Value Of Chlorine The greater the attractive forces between the. Electron affinity of chlorine is 349 kj/mol. The values of electron affinity are given in kj/mol. 119 rows periodic table with electron affinity values is shown above. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. As the name suggests, electron affinity is the. Electron Affinity Value Of Chlorine.
From brokeasshome.com
Periodic Table Chlorine Electrons Electron Affinity Value Of Chlorine In chemistry and atomic physics, the electron affinity of an atom or molecule is. The values of electron affinity are given in kj/mol. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity of chlorine. Electron Affinity Value Of Chlorine.
From hohpasap.weebly.com
What is electron affinity hohpasap Electron Affinity Value Of Chlorine In chemistry and atomic physics, the electron affinity of an atom or molecule is. Values in parentheses ( ) are predicted values. Simply speaking, the greater the affinity of electrons, the more. By convention, the negative sign shows a release of energy. As the name suggests, electron affinity is the ability of an atom to accept an electron. The value. Electron Affinity Value Of Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Value Of Chlorine By convention, the negative sign shows a release of energy. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The chlorine atom has the most negative electron affinity of any element, which means that more energy is. Electron Affinity Value Of Chlorine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Electron Affinity Value Of Chlorine The greater the attractive forces between the. Electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. 103 rows electron affinity is related to electronegativity. Electron Affinity Value Of Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Value Of Chlorine The greater the attractive forces between the. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. 103 rows electron affinity is related to electronegativity of elements. 119 rows periodic table with electron affinity values is shown above.. Electron Affinity Value Of Chlorine.
From www.toppr.com
The electron affinity of chlorine is 3.7 eV. 1 gram of chlorine is Electron Affinity Value Of Chlorine 103 rows electron affinity is related to electronegativity of elements. The values of electron affinity are given in kj/mol. Electron affinity of chlorine is 349 kj/mol. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. The. Electron Affinity Value Of Chlorine.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Electron Affinity Value Of Chlorine Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Electron affinity of chlorine is 349 kj/mol. The greater the attractive forces between the. Unlike electronegativity, electron affinity. Electron Affinity Value Of Chlorine.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Electron Affinity Value Of Chlorine As the name suggests, electron affinity is the ability of an atom to accept an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. The value of the electron affinity depends on how strongly the. Electron Affinity Value Of Chlorine.
From ar.inspiredpencil.com
Electron Affinity Values Electron Affinity Value Of Chlorine 103 rows electron affinity is related to electronegativity of elements. As the name suggests, electron affinity is the ability of an atom to accept an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Values. Electron Affinity Value Of Chlorine.
From study.com
Electron Affinity Definition, Trends & Equation Video & Lesson Electron Affinity Value Of Chlorine The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Unlike electronegativity, electron affinity is a. Simply speaking, the greater the affinity of electrons, the more. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Electron Affinity Value Of Chlorine.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Electron Affinity Value Of Chlorine Simply speaking, the greater the affinity of electrons, the more. As the name suggests, electron affinity is the ability of an atom to accept an electron. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. By convention, the negative sign shows a release of energy.. Electron Affinity Value Of Chlorine.
From mavink.com
Periodic Table With Electron Affinity Electron Affinity Value Of Chlorine Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. The greater the attractive forces between the. 103 rows electron affinity is related to electronegativity of elements. Values in parentheses ( ) are predicted values. Electron affinity of chlorine is 349 kj/mol. In chemistry and atomic. Electron Affinity Value Of Chlorine.
From driverlayer.com
electron affinity DriverLayer Search Engine Electron Affinity Value Of Chlorine 103 rows electron affinity is related to electronegativity of elements. By convention, the negative sign shows a release of energy. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. The value of the electron affinity depends on how strongly the incoming electron is attracted to. Electron Affinity Value Of Chlorine.
From www.toppr.com
The electron affinity of chlorine is 3.7eV . How much energy (nearest Electron Affinity Value Of Chlorine Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. As the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity,. Electron Affinity Value Of Chlorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Electron Affinity Value Of Chlorine By convention, the negative sign shows a release of energy. As the name suggests, electron affinity is the ability of an atom to accept an electron. The values of electron affinity are given in kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The chlorine atom has the most negative electron affinity of any. Electron Affinity Value Of Chlorine.
From www.toppr.com
14. Which of the (1) Phosphor which of the following statement is/are Electron Affinity Value Of Chlorine The greater the attractive forces between the. 119 rows periodic table with electron affinity values is shown above. Values in parentheses ( ) are predicted values. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. 103 rows electron affinity is related to electronegativity of elements. As the name suggests, electron affinity. Electron Affinity Value Of Chlorine.
From gbu-taganskij.ru
Electron Affinity Definition, Chart Trend In Periodic, 47 OFF Electron Affinity Value Of Chlorine 119 rows periodic table with electron affinity values is shown above. Simply speaking, the greater the affinity of electrons, the more. The values of electron affinity are given in kj/mol. Values in parentheses ( ) are predicted values. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Electron affinity of chlorine. Electron Affinity Value Of Chlorine.
From byjus.com
6. d the electron affinity of chlorine from the following data Electron Affinity Value Of Chlorine The greater the attractive forces between the. 103 rows electron affinity is related to electronegativity of elements. 119 rows periodic table with electron affinity values is shown above. Electron affinity of chlorine is 349 kj/mol. The values of electron affinity are given in kj/mol. By convention, the negative sign shows a release of energy. Energy is released when the first. Electron Affinity Value Of Chlorine.
From socratic.org
Which following pairs of atoms, have a lower electron affinity? a) Ca,K Electron Affinity Value Of Chlorine Simply speaking, the greater the affinity of electrons, the more. 119 rows periodic table with electron affinity values is shown above. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. The values of electron affinity are given in kj/mol. Electron affinity of chlorine is 349 kj/mol. The chlorine atom has the. Electron Affinity Value Of Chlorine.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Electron Affinity Value Of Chlorine 119 rows periodic table with electron affinity values is shown above. In chemistry and atomic physics, the electron affinity of an atom or molecule is. By convention, the negative sign shows a release of energy. The values of electron affinity are given in kj/mol. The greater the attractive forces between the. The chlorine atom has the most negative electron affinity. Electron Affinity Value Of Chlorine.
From www.pearson.com
The periodic table with electron affinity values is shown below Electron Affinity Value Of Chlorine As the name suggests, electron affinity is the ability of an atom to accept an electron. Values in parentheses ( ) are predicted values. 119 rows periodic table with electron affinity values is shown above. By convention, the negative sign shows a release of energy. The greater the attractive forces between the. Unlike electronegativity, electron affinity is a. The chlorine. Electron Affinity Value Of Chlorine.
From ar.inspiredpencil.com
Electron Affinity List Electron Affinity Value Of Chlorine The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. In chemistry and atomic physics, the electron affinity of an atom or molecule is. As the name suggests, electron affinity is the ability of an atom to. Electron Affinity Value Of Chlorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Electron Affinity Value Of Chlorine 119 rows periodic table with electron affinity values is shown above. In chemistry and atomic physics, the electron affinity of an atom or molecule is. As the name suggests, electron affinity is the ability of an atom to accept an electron. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Values. Electron Affinity Value Of Chlorine.
From socratic.org
Why is the election affinity of beryllium negative? Socratic Electron Affinity Value Of Chlorine As the name suggests, electron affinity is the ability of an atom to accept an electron. 119 rows periodic table with electron affinity values is shown above. Unlike electronegativity, electron affinity is a. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Energy is released when the first electron is added to an atom and. Electron Affinity Value Of Chlorine.
From www.toppr.com
Calculate the electron affinity of fluorine atom using the following Electron Affinity Value Of Chlorine Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Values in parentheses ( ) are predicted values. Electron affinity of chlorine is 349 kj/mol. Unlike electronegativity, electron affinity is a. The. Electron Affinity Value Of Chlorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Value Of Chlorine The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. 103 rows electron affinity is related to electronegativity of elements. By convention, the negative sign shows a release of energy. As the name suggests, electron affinity is. Electron Affinity Value Of Chlorine.
From www.breakingatom.com
Electron Affinity of The Elements Electron Affinity Value Of Chlorine 119 rows periodic table with electron affinity values is shown above. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of chlorine is 349 kj/mol. The values of electron affinity are given in kj/mol. Energy is released when the first electron is added to an atom and monovalent anion is formed and this. Electron Affinity Value Of Chlorine.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Electron Affinity Value Of Chlorine The greater the attractive forces between the. Energy is released when the first electron is added to an atom and monovalent anion is formed and this is known as the first. By convention, the negative sign shows a release of energy. Simply speaking, the greater the affinity of electrons, the more. 103 rows electron affinity is related to electronegativity of. Electron Affinity Value Of Chlorine.
From www.chegg.com
Solved Calcium has a greater electron affinity than Chlorine Electron Affinity Value Of Chlorine By convention, the negative sign shows a release of energy. Values in parentheses ( ) are predicted values. The values of electron affinity are given in kj/mol. Simply speaking, the greater the affinity of electrons, the more. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is. Electron Affinity Value Of Chlorine.
From scienceinfo.com
Electron affinity Electron Affinity Value Of Chlorine The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity of chlorine is 349 kj/mol. By convention, the negative sign shows a release of energy. The value of the electron affinity depends on how strongly. Electron Affinity Value Of Chlorine.
From www.savemyexams.com
Electron Affinity & Trends of Group 16 & 17 Elements CIE A Level Electron Affinity Value Of Chlorine By convention, the negative sign shows a release of energy. The values of electron affinity are given in kj/mol. Electron affinity of chlorine is 349 kj/mol. 119 rows periodic table with electron affinity values is shown above. The greater the attractive forces between the. 103 rows electron affinity is related to electronegativity of elements. The value of the electron affinity. Electron Affinity Value Of Chlorine.
From wisc.pb.unizin.org
D4.4 Periodic Variation in Electron Affinities Chem 109 Fall 2023 Electron Affinity Value Of Chlorine Unlike electronegativity, electron affinity is a. As the name suggests, electron affinity is the ability of an atom to accept an electron. By convention, the negative sign shows a release of energy. The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus. Simply speaking, the greater the affinity of electrons, the more.. Electron Affinity Value Of Chlorine.