How Many Valence Electrons Do Bromine Have . 119 rows valence electrons: Similar to iodine, all elements in this group have 7. Bromine is a halogen element and its symbol is ‘br’. Thus, bromine has seven valence electrons. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). There are two ways to find the number of valence electrons in bromine (br). 93 rows updated on may 19, 2024. The first is to use the. You may assume the valences of the chemical elements—the number of electrons with which. The valence electrons are the total number of electrons in the last orbit (shell). Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The total number of electrons in the last.
from www.numerade.com
The total number of electrons in the last. The first is to use the. 93 rows updated on may 19, 2024. The valence electrons are the total number of electrons in the last orbit (shell). Similar to iodine, all elements in this group have 7. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. There are two ways to find the number of valence electrons in bromine (br). You may assume the valences of the chemical elements—the number of electrons with which. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. Bromine is a halogen element and its symbol is ‘br’.
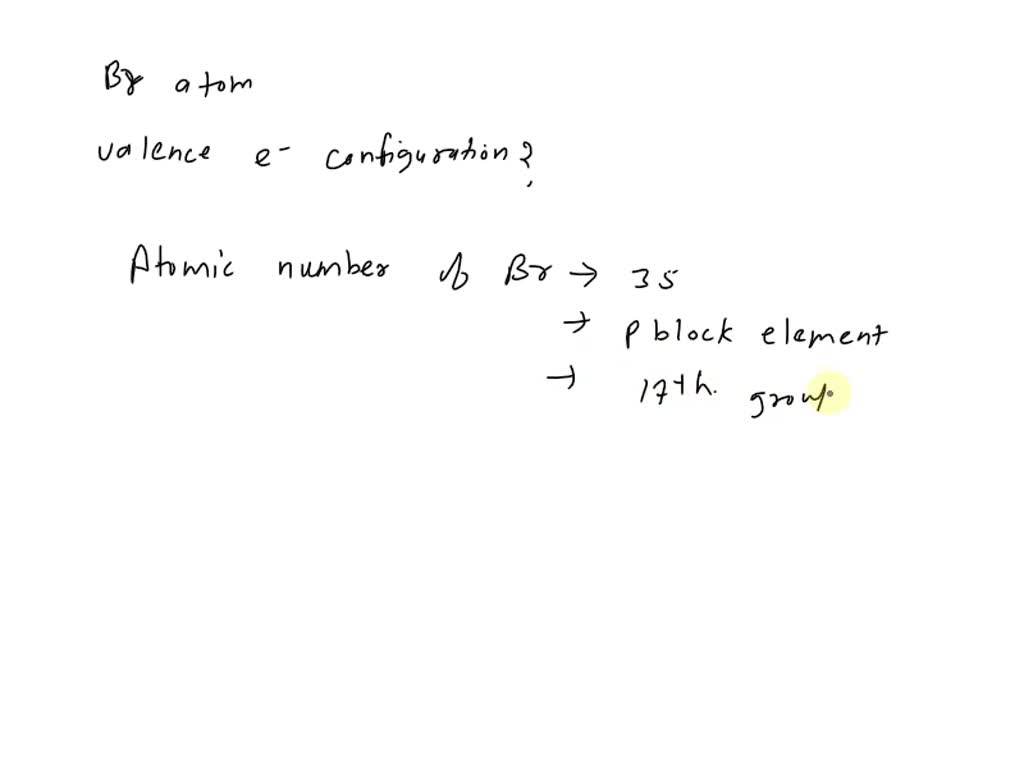
SOLVED What is the valence electron configuration for the bromine atom?
How Many Valence Electrons Do Bromine Have There are two ways to find the number of valence electrons in bromine (br). Bromine is a halogen element and its symbol is ‘br’. There are two ways to find the number of valence electrons in bromine (br). 93 rows updated on may 19, 2024. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. You may assume the valences of the chemical elements—the number of electrons with which. Thus, bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The valence electrons are the total number of electrons in the last orbit (shell). The first is to use the. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. 119 rows valence electrons: Similar to iodine, all elements in this group have 7. The total number of electrons in the last.
From phootoscelebrities.com
How many valence electrons does bromine have? How Many Valence Electrons Do Bromine Have You may assume the valences of the chemical elements—the number of electrons with which. Similar to iodine, all elements in this group have 7. Thus, bromine has seven valence electrons. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. There are two. How Many Valence Electrons Do Bromine Have.
From study.com
Valence Electrons Definition, Role & Examples Video & Lesson How Many Valence Electrons Do Bromine Have 93 rows updated on may 19, 2024. Similar to iodine, all elements in this group have 7. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence. How Many Valence Electrons Do Bromine Have.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine How Many Valence Electrons Do Bromine Have Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The first is to use the. 93 rows updated on may 19, 2024. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. 119 rows valence. How Many Valence Electrons Do Bromine Have.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons How Many Valence Electrons Do Bromine Have 119 rows valence electrons: The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The valence electrons are the total number of electrons in the last orbit (shell). Thus, bromine has seven valence electrons. There. How Many Valence Electrons Do Bromine Have.
From www.youtube.com
How many valence electrons does bromine have? YouTube How Many Valence Electrons Do Bromine Have Thus, bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons. How Many Valence Electrons Do Bromine Have.
From www.youtube.com
Number of Valence Electrons for Hydrogen (H) YouTube How Many Valence Electrons Do Bromine Have The valence electrons are the total number of electrons in the last orbit (shell). Thus, bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). 119 rows valence electrons: Bromine. How Many Valence Electrons Do Bromine Have.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo How Many Valence Electrons Do Bromine Have The first is to use the. The total number of electrons in the last. Thus, bromine has seven valence electrons. The valence electrons are the total number of electrons in the last orbit (shell). 119 rows valence electrons: The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4. How Many Valence Electrons Do Bromine Have.
From phootoscelebrities.com
How Many Valence Electrons Does Bromine Have? How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The total number of electrons in the last. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. How Many Valence Electrons Do Bromine Have.
From www.inspiritvr.com
Valence Electrons In Beryllium Study Guide Inspirit How Many Valence Electrons Do Bromine Have 93 rows updated on may 19, 2024. Similar to iodine, all elements in this group have 7. The total number of electrons in the last. You may assume the valences of the chemical elements—the number of electrons with which. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in. How Many Valence Electrons Do Bromine Have.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of How Many Valence Electrons Do Bromine Have The first is to use the. The valence electrons are the total number of electrons in the last orbit (shell). Bromine is a halogen element and its symbol is ‘br’. 93 rows updated on may 19, 2024. The total number of electrons in the last. Similar to iodine, all elements in this group have 7. There are two ways to. How Many Valence Electrons Do Bromine Have.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. The total number of electrons in the last. Similar to iodine, all elements in this group have 7. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
Silver(Ag) electron configuration and orbital diagram How Many Valence Electrons Do Bromine Have The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. You may assume the valences of the chemical elements—the number of electrons with which. Bromine is a halogen element and its symbol is ‘br’. The first is to use the. Bromine is located. How Many Valence Electrons Do Bromine Have.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic How Many Valence Electrons Do Bromine Have Thus, bromine has seven valence electrons. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last. The valence electrons are the sum of the electrons in the outermost shell, that is. How Many Valence Electrons Do Bromine Have.
From material-properties.org
Bromine Periodic Table and Atomic Properties How Many Valence Electrons Do Bromine Have 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. 119 rows valence electrons: The valence electrons are the total number of electrons in the last orbit (shell). Thus, bromine has seven valence electrons. Bromine is a halogen element and its symbol is ‘br’. Similar to iodine, all elements. How Many Valence Electrons Do Bromine Have.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Many Valence Electrons Do Bromine Have 93 rows updated on may 19, 2024. 119 rows valence electrons: The total number of electrons in the last. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. You may assume the valences of the chemical elements—the number of electrons with which.. How Many Valence Electrons Do Bromine Have.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. The first is to use the. You may assume the valences of the chemical elements—the number of electrons with which. 119 rows valence electrons: 93 rows updated on may 19, 2024. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. Similar to. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
How to Find the Valence Electrons for COCl2 (phosgene)? How Many Valence Electrons Do Bromine Have The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. You may assume the valences of the chemical elements—the number of electrons with which. The valence electrons are the total number of electrons in the last orbit (shell). 119 rows valence electrons: Bromine. How Many Valence Electrons Do Bromine Have.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare How Many Valence Electrons Do Bromine Have The total number of electrons in the last. The first is to use the. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. There are two ways to find the number of valence electrons in bromine (br). Thus, bromine has seven valence. How Many Valence Electrons Do Bromine Have.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram How Many Valence Electrons Do Bromine Have The valence electrons are the total number of electrons in the last orbit (shell). 93 rows updated on may 19, 2024. Similar to iodine, all elements in this group have 7. 119 rows valence electrons: Bromine is a halogen element and its symbol is ‘br’. There are two ways to find the number of valence electrons in bromine (br). Bromine. How Many Valence Electrons Do Bromine Have.
From mungfali.com
Dynamic Periodic Table Of Elements How Many Valence Electrons Do Bromine Have The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last. Thus, bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). Similar to iodine, all elements in this group have 7. The total number of electrons present in the. How Many Valence Electrons Do Bromine Have.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. There are two ways to find the number of valence electrons in bromine (br). The first is to use the. The total number of electrons in the last. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. 119 rows valence electrons: 93. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
How Many Valence Electrons Does BrF5 Have? How Many Valence Electrons Do Bromine Have The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last. The valence electrons. How Many Valence Electrons Do Bromine Have.
From www.toppr.com
How many valence electrons does cadmium have? How Many Valence Electrons Do Bromine Have The valence electrons are the total number of electrons in the last orbit (shell). Bromine is a halogen element and its symbol is ‘br’. You may assume the valences of the chemical elements—the number of electrons with which. There are two ways to find the number of valence electrons in bromine (br). The first is to use the. Similar to. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
How Many Valence Electrons Does Oxygen (O) Have? How Many Valence Electrons Do Bromine Have There are two ways to find the number of valence electrons in bromine (br). Thus, bromine has seven valence electrons. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. 119 rows valence electrons: The total number of electrons in the last. The. How Many Valence Electrons Do Bromine Have.
From brokeasshome.com
Periodic Table Of Elements With Atomic Mass And Valency How Many Valence Electrons Do Bromine Have 93 rows updated on may 19, 2024. 119 rows valence electrons: Bromine is a halogen element and its symbol is ‘br’. Similar to iodine, all elements in this group have 7. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last. The total number of electrons present in. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) How Many Valence Electrons Do Bromine Have The valence electrons are the total number of electrons in the last orbit (shell). Thus, bromine has seven valence electrons. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. Similar to iodine, all elements in this group have 7. You may assume the valences of the chemical elements—the number of electrons with. How Many Valence Electrons Do Bromine Have.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? How Many Valence Electrons Do Bromine Have 119 rows valence electrons: The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. Similar to iodine, all elements in this group have 7. Thus, bromine has seven valence electrons. You may assume the valences of the chemical elements—the number of electrons with. How Many Valence Electrons Do Bromine Have.
From www.youtube.com
How many valence electrons does bromine have? YouTube How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. You may assume the valences of the chemical elements—the number of electrons with which. 93 rows updated on may 19, 2024. There are two ways to find the number of valence electrons in bromine (br). The valence electrons are the total number of electrons in the last orbit (shell). The. How Many Valence Electrons Do Bromine Have.
From www.numerade.com
SOLVEDWrite out the groundstate electron configuration for the How Many Valence Electrons Do Bromine Have Thus, bromine has seven valence electrons. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. You may assume the valences of the chemical elements—the number. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
How many valence electrons does cobalt(Co) have? How Many Valence Electrons Do Bromine Have There are two ways to find the number of valence electrons in bromine (br). You may assume the valences of the chemical elements—the number of electrons with which. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The total number of electrons in the last. The first is to use the. Bromine. How Many Valence Electrons Do Bromine Have.
From proper-cooking.info
Bromine Valence Electrons How Many Valence Electrons Do Bromine Have Bromine is a halogen element and its symbol is ‘br’. Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. 119 rows valence electrons: Thus, bromine has seven valence electrons. The first is to use the. The valence electrons are the total number of electrons in the last orbit (shell). The valence electrons. How Many Valence Electrons Do Bromine Have.
From valenceelectrons.com
How many valence electrons does bromine(Br) have? How Many Valence Electrons Do Bromine Have The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. The valence electrons are the total number of electrons in the last orbit (shell). There are two ways to find the number of valence electrons in bromine (br). The total number of electrons. How Many Valence Electrons Do Bromine Have.
From scientifictutor.org
Chem Valence Electrons Scientific Tutor How Many Valence Electrons Do Bromine Have The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Bromine is located in the halogens family, which is group 17 (or viia) of the periodic table. The valence electrons are the total number of. How Many Valence Electrons Do Bromine Have.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic How Many Valence Electrons Do Bromine Have 119 rows valence electrons: The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a. The valence electrons are the total number of electrons in the last orbit (shell). There are two ways to find the number of valence electrons in bromine (br). Bromine. How Many Valence Electrons Do Bromine Have.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia How Many Valence Electrons Do Bromine Have 119 rows valence electrons: You may assume the valences of the chemical elements—the number of electrons with which. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the. How Many Valence Electrons Do Bromine Have.