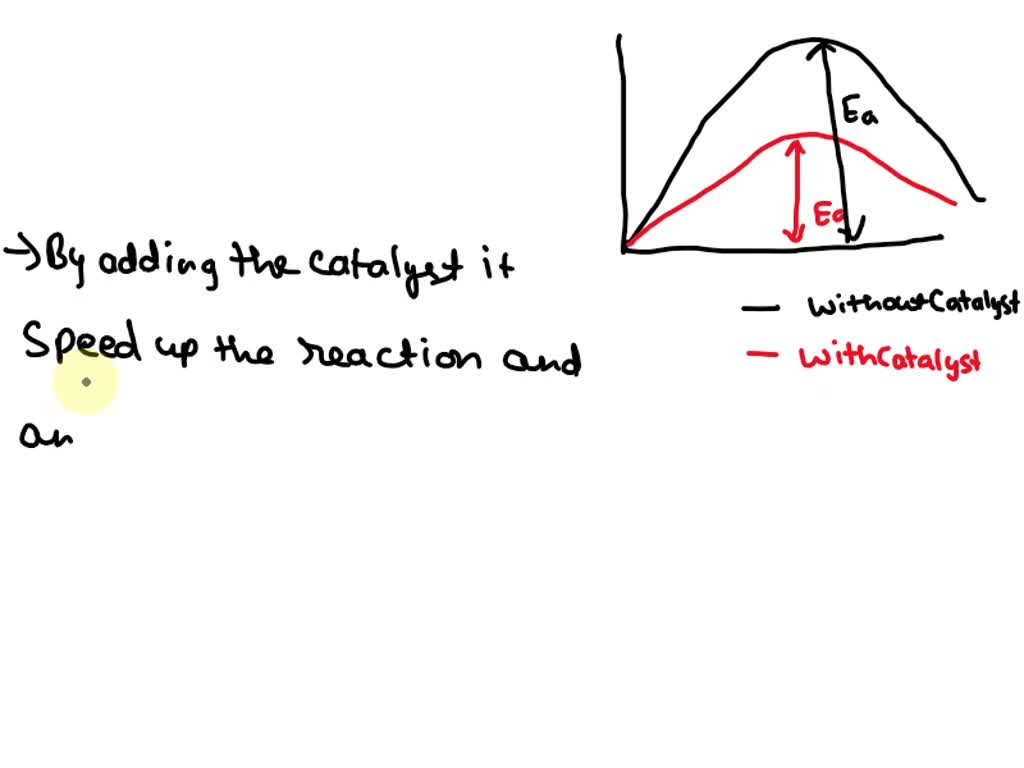
How Does A Catalyst Increase The Rate Of A Reaction Quizlet . This page explains how adding a catalyst affects the rate of a reaction. Thus collisions that wouldn't provide enough. Mass is measured in kilograms (kg) or grams (g). Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. How does a catalyst increase the rate of a reaction? A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. Of catalyst is needed to increase the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. It increases the energy difference between the reactants and products. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts. It does so by providing an alternative reaction pathway.
from www.numerade.com
A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. Thus collisions that wouldn't provide enough. However, not all reactions have suitable catalysts. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Different substances catalyse different reactions. How does a catalyst increase the rate of a reaction? Mass is measured in kilograms (kg) or grams (g). It increases the energy difference between the reactants and products. It does so by providing an alternative reaction pathway. How does a catalyst increase the rate of a reaction?
SOLVED How does a catalyst increase the speed of a reaction? A. The
How Does A Catalyst Increase The Rate Of A Reaction Quizlet This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. It increases the energy difference between the reactants and products. However, not all reactions have suitable catalysts. It does so by providing an alternative reaction pathway. How does a catalyst increase the rate of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Mass is measured in kilograms (kg) or grams (g). How does a catalyst increase the rate of a reaction? Only a very small mass of catalyst is needed to increase the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Thus collisions that wouldn't provide enough.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii How Does A Catalyst Increase The Rate Of A Reaction Quizlet Mass is measured in kilograms (kg) or grams (g). A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts. This page explains how adding a catalyst affects the rate of a reaction. A catalyst speeds up. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVEDHow does a catalyst increase the rate of a chemical reaction? How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Different substances catalyse different reactions. Thus collisions that wouldn't provide enough. This page describes and explains the way that. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog How Does A Catalyst Increase The Rate Of A Reaction Quizlet It does so by providing an alternative reaction pathway. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From askfilo.com
How does a catalyst increase the rate of a reaction 3 Filo How Does A Catalyst Increase The Rate Of A Reaction Quizlet Only a very small mass of catalyst is needed to increase the rate of a reaction. It increases the energy difference between the reactants and products. However, not all reactions have suitable catalysts. Mass is measured in kilograms (kg) or grams (g). A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 How Does A Catalyst Increase The Rate Of A Reaction Quizlet Of catalyst is needed to increase the rate of a reaction. It increases the energy difference between the reactants and products. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? It does so by providing. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Does A Catalyst Increase The Rate Of A Reaction Quizlet It does so by providing an alternative reaction pathway. How does a catalyst increase the rate of a reaction? Of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy.. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. It increases the energy difference between the reactants and products. Mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. How does a catalyst increase the rate of a reaction? It assumes that you are already familiar with basic ideas about the collision. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.slideshare.net
Rate of reaction(f5) How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. Thus collisions that wouldn't provide enough. Different substances catalyse different reactions. How does a catalyst increase the rate of a reaction? It does so by providing an alternative reaction pathway. Mass is measured in kilograms (kg) or grams (g). How does a catalyst increase the rate of a reaction? This page explains how. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.gauthmath.com
Solved How does a catalyst increase the rate of a chemical reaction How Does A Catalyst Increase The Rate Of A Reaction Quizlet Thus collisions that wouldn't provide enough. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. How does a catalyst increase the rate of a reaction? Mass is measured in kilograms (kg) or grams (g). Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.sasadoctor.com
Rate of reaction chemistry igcse How Does A Catalyst Increase The Rate Of A Reaction Quizlet Mass is measured in kilograms (kg) or grams (g). Different substances catalyse different reactions. However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. It does so by providing. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From byjus.com
How does a catalyst increase the rate of a reaction? How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Of catalyst is needed to increase the rate of a reaction. It does so by providing an alternative reaction pathway. Only a very small mass of. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a How Does A Catalyst Increase The Rate Of A Reaction Quizlet Only a very small mass of catalyst is needed to increase the rate of a reaction. How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Mass is measured in kilograms (kg) or grams (g). Of. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVEDHow does a catalyst increase the rate of a reaction? How Does A Catalyst Increase The Rate Of A Reaction Quizlet A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. It does so by providing an alternative reaction pathway. Different substances catalyse different reactions. This page explains how adding a catalyst affects the rate of a reaction. How does a catalyst increase the rate of a. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.chegg.com
Solved 12. How does a catalyst increase the rate of a How Does A Catalyst Increase The Rate Of A Reaction Quizlet Thus collisions that wouldn't provide enough. Of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. It assumes that you are already familiar with basic. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Does A Catalyst Increase The Rate Of A Reaction Quizlet Thus collisions that wouldn't provide enough. This page explains how adding a catalyst affects the rate of a reaction. How does a catalyst increase the rate of a reaction? Only a very small mass of catalyst is needed to increase the rate of a reaction. Different substances catalyse different reactions. It increases the energy difference between the reactants and products.. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change How Does A Catalyst Increase The Rate Of A Reaction Quizlet A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Different substances catalyse different reactions. However, not all reactions have suitable catalysts. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. Only a very small mass of catalyst. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.chegg.com
Solved How does a catalyst increase the rate of a reaction? How Does A Catalyst Increase The Rate Of A Reaction Quizlet It increases the energy difference between the reactants and products. However, not all reactions have suitable catalysts. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. This page explains how adding a catalyst affects the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. Thus collisions. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology How Does A Catalyst Increase The Rate Of A Reaction Quizlet How does a catalyst increase the rate of a reaction? Of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry How Does A Catalyst Increase The Rate Of A Reaction Quizlet How does a catalyst increase the rate of a reaction? Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From quizlet.com
4 Main factors that affect the rate of chemical reactions Diagram Quizlet How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. It increases the energy difference between the reactants and products. Thus collisions. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From loeljyzzv.blob.core.windows.net
Catalyst Chemical Reaction Rate at Leandro Jordan blog How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. It does so by providing an alternative reaction pathway. Only a very small mass of catalyst is needed to increase the rate of a reaction. This. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Does A Catalyst Increase The Rate Of A Reaction Quizlet This page describes and explains the way that adding a catalyst affects the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. However, not all reactions have suitable catalysts. Thus. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From askfilo.com
1. How catalyst increase the rate of reaction? Explain it by graph2. Exp.. How Does A Catalyst Increase The Rate Of A Reaction Quizlet This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Only a very small mass of catalyst is needed to increase the rate. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions How Does A Catalyst Increase The Rate Of A Reaction Quizlet Different substances catalyse different reactions. How does a catalyst increase the rate of a reaction? This page explains how adding a catalyst affects the rate of a reaction. How does a catalyst increase the rate of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Only a very small mass. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVED Quick Question The reaction required a catalyst; HCI What is a How Does A Catalyst Increase The Rate Of A Reaction Quizlet Different substances catalyse different reactions. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Of catalyst is needed to increase the rate of a reaction. How does a catalyst increase the rate of a reaction? How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts.. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Does A Catalyst Increase The Rate Of A Reaction Quizlet Of catalyst is needed to increase the rate of a reaction. It increases the energy difference between the reactants and products. It does so by providing an alternative reaction pathway. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVED How does a catalyst increase the speed of a reaction? A. The How Does A Catalyst Increase The Rate Of A Reaction Quizlet It increases the energy difference between the reactants and products. How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Thus collisions that wouldn't provide enough. It assumes that. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
SOLVED can you solve ? Regarding to catalysts, how does a catalyst How Does A Catalyst Increase The Rate Of A Reaction Quizlet Different substances catalyse different reactions. How does a catalyst increase the rate of a reaction? However, not all reactions have suitable catalysts. However, not all reactions have suitable catalysts. It increases the energy difference between the reactants and products. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript How Does A Catalyst Increase The Rate Of A Reaction Quizlet It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. However, not all reactions have suitable catalysts. Thus collisions that wouldn't provide enough. How does a catalyst increase the rate of a reaction? A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Different substances catalyse. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From joijjhybw.blob.core.windows.net
Catalyst Affect Rate Of Reaction at Carmelo Gentry blog How Does A Catalyst Increase The Rate Of A Reaction Quizlet It does so by providing an alternative reaction pathway. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. Only a very small mass of catalyst is needed to increase the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. It increases the energy difference between the. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 How Does A Catalyst Increase The Rate Of A Reaction Quizlet However, not all reactions have suitable catalysts. Thus collisions that wouldn't provide enough. However, not all reactions have suitable catalysts. Mass is measured in kilograms (kg) or grams (g). This page describes and explains the way that adding a catalyst affects the rate of a reaction. Different substances catalyse different reactions. Only a very small mass of catalyst is needed. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.numerade.com
⏩SOLVEDWhy does a catalyst increase the rate of a reaction? What is How Does A Catalyst Increase The Rate Of A Reaction Quizlet It assumes that you are already familiar with basic ideas about the collision theory of reaction rates,. Of catalyst is needed to increase the rate of a reaction. Thus collisions that wouldn't provide enough. However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? It does so by providing an alternative reaction pathway.. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes How Does A Catalyst Increase The Rate Of A Reaction Quizlet This page describes and explains the way that adding a catalyst affects the rate of a reaction. It does so by providing an alternative reaction pathway. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. How does a catalyst increase the rate of a reaction? Of. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.chegg.com
Solved s. How does a catalyst increase the rate of a How Does A Catalyst Increase The Rate Of A Reaction Quizlet This page explains how adding a catalyst affects the rate of a reaction. It does so by providing an alternative reaction pathway. A catalyst speeds up a reaction by providing a set of elementary steps with more favorable kinetics than those that exist in its absence. Only a very small mass of catalyst is needed to increase the rate of. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.
From www.sasadoctor.com
Rate of reaction chemistry igcse How Does A Catalyst Increase The Rate Of A Reaction Quizlet It increases the energy difference between the reactants and products. Thus collisions that wouldn't provide enough. Mass is measured in kilograms (kg) or grams (g). This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a reaction by lowering the activation energy. How does a catalyst increase. How Does A Catalyst Increase The Rate Of A Reaction Quizlet.