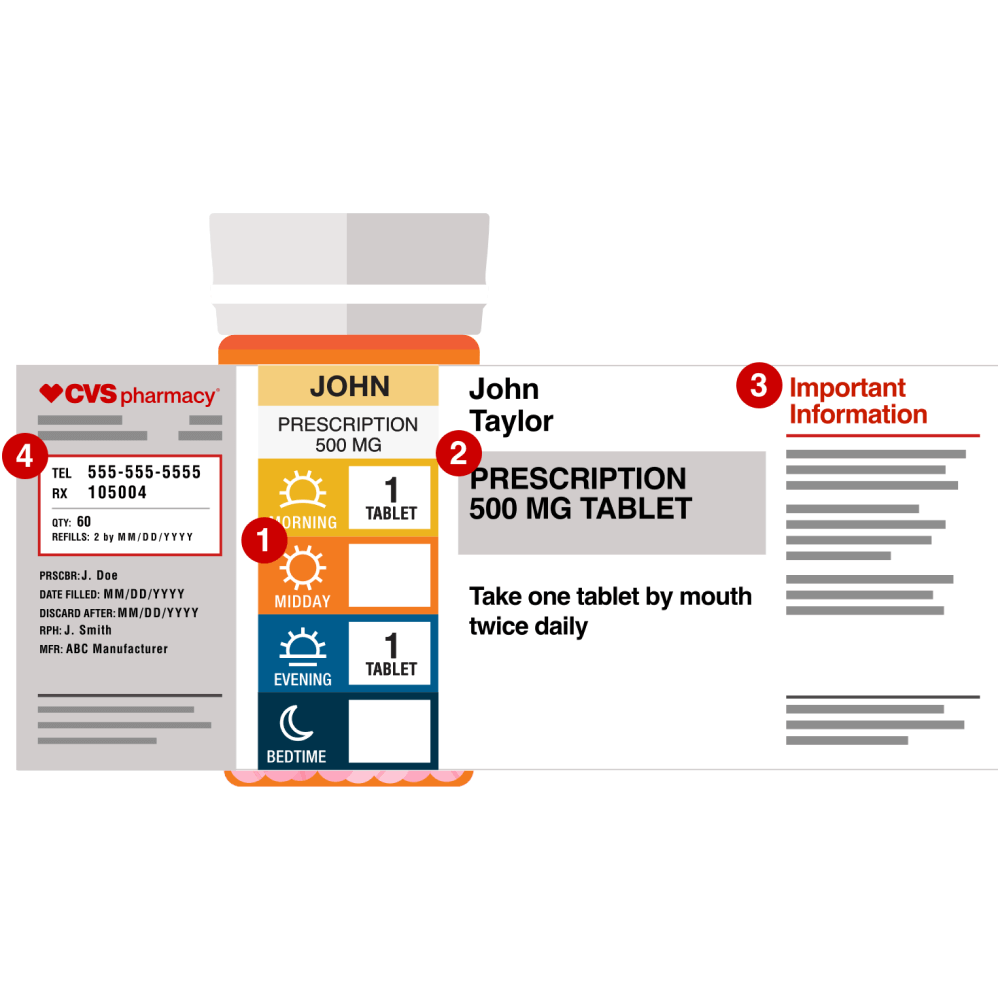
Ocp Prescription Label Requirements . The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling of drugs dispensed on prescription. Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or.
from www.vrogue.co
(a) no drug may be dispensed by outpatient prescription unless a. Or if brand name were to be used: Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or.
Prescription Bottle Label Template Download Template vrogue.co
Ocp Prescription Label Requirements Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed on prescription.
From giomaacex.blob.core.windows.net
Purposes Of Label Uses Of A Product With Relevant Examples at Gerald Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling. Ocp Prescription Label Requirements.
From exoduzryz.blob.core.windows.net
Fda Label Guidance at Debra Jumper blog Ocp Prescription Label Requirements Or if brand name were to be used: A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below. Ocp Prescription Label Requirements.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Labeling of drugs dispensed on prescription. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to be used: A product comprised of two or more regulated components. Ocp Prescription Label Requirements.
From old.sermitsiaq.ag
Veterinary Prescription Template Ocp Prescription Label Requirements A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Or if brand name were to be used: Labeling of drugs dispensed on prescription. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription unless a. Office of. Ocp Prescription Label Requirements.
From www.studypool.com
SOLUTION OCP Prescription Regulation Summary Chart Studypool Ocp Prescription Label Requirements Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. (a) no drug may be dispensed by outpatient prescription unless a. A product comprised of. Ocp Prescription Label Requirements.
From www.researchgate.net
Odds ratio of filling a brandname OCP prescription relative to generic Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription unless a. A product comprised of two or. Ocp Prescription Label Requirements.
From oliverdesign.com
Unfolding Prescription Drug Labeling Requirements Oliver Design Ocp Prescription Label Requirements (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed on prescription. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to be used: A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Prescription Medicine Label Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. A product comprised of two or. Ocp Prescription Label Requirements.
From healthyheels.org
prescription drug UNC Healthy Heels Ocp Prescription Label Requirements (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device,. Ocp Prescription Label Requirements.
From www.lifealert.org
OvertheCounter Medicine Label Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to. Ocp Prescription Label Requirements.
From www.scribd.com
5014Prescription Regulation Table PDF Medical Prescription Pharmacy Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. Or if brand name were to be used: Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription. Ocp Prescription Label Requirements.
From doctorlib.info
ORAL CONTRACEPTIVEMONOPHASIC Various Top 300 Pharmacy Drug Cards Ocp Prescription Label Requirements (a) no drug may be dispensed by outpatient prescription unless a. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling of drugs dispensed. Ocp Prescription Label Requirements.
From etactics.com
Prescription Label Design Why It Matters and Effective Examples — Etactics Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to be used: Labeling of drugs dispensed. Ocp Prescription Label Requirements.
From www.youtube.com
How to read a medication label YouTube Ocp Prescription Label Requirements The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling of drugs dispensed on prescription. Or if brand name were to be used: Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components. Ocp Prescription Label Requirements.
From giocirsmu.blob.core.windows.net
Drug Facts Box Requirements at Deleon blog Ocp Prescription Label Requirements A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to be used: Labeling. Ocp Prescription Label Requirements.
From www.federalregister.gov
Federal Register OvertheCounter Vaginal Contraceptive and Ocp Prescription Label Requirements A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity. Ocp Prescription Label Requirements.
From www.vrogue.co
Prescription Bottle Label Template Download Template vrogue.co Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: Labeling of drugs dispensed on prescription. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. (a) no drug may be dispensed by outpatient prescription unless a. The resources below. Ocp Prescription Label Requirements.
From www.vrogue.co
Sample Drug Facts Label Fda vrogue.co Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Ocp Prescription Label Requirements Or if brand name were to be used: Labeling of drugs dispensed on prescription. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components. Ocp Prescription Label Requirements.
From www.studocu.com
Week 4 Prescription label requirements study guide 2022 PHM 322 Week Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. Or if brand name were to. Ocp Prescription Label Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Ocp Prescription Label Requirements Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. The resources below. Ocp Prescription Label Requirements.
From www.medscape.com
Introduction to the New Prescription Drug Labeling by the FDA Ocp Prescription Label Requirements (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed on prescription. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to. Ocp Prescription Label Requirements.
From www.researchgate.net
(PDF) Drug labeling The study of compliance of regulatory requirements Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed. Ocp Prescription Label Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Ocp Prescription Label Requirements Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. Labeling of drugs dispensed on prescription. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Prescription Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription unless a. A product comprised of. Ocp Prescription Label Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Ocp Prescription Label Requirements A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug. Ocp Prescription Label Requirements.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Ocp Prescription Label Requirements The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Or if brand name were to be used: (a) no drug may be dispensed by outpatient prescription unless a. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Labeling of drugs dispensed on prescription. Office of. Ocp Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Ocp Prescription Label Requirements Labeling of drugs dispensed on prescription. (a) no drug may be dispensed by outpatient prescription unless a. Or if brand name were to be used: The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of. Ocp Prescription Label Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Ocp Prescription Label Requirements (a) no drug may be dispensed by outpatient prescription unless a. Or if brand name were to be used: Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. Labeling of drugs dispensed on prescription. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. The resources below. Ocp Prescription Label Requirements.
From www.federalregister.gov
Federal Register Requirements on Content and Format of Labeling for Ocp Prescription Label Requirements The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Or if brand name were to be used: Labeling. Ocp Prescription Label Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Ocp Prescription Label Requirements A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. Labeling of drugs dispensed on prescription. Or if brand name were to be used: Office of. Ocp Prescription Label Requirements.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Ocp Prescription Label Requirements The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. (a) no drug may be dispensed by outpatient prescription unless a. Or if brand name were to be used: A product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or. Office of communications division of drug information center. Ocp Prescription Label Requirements.
From dailymed.nlm.nih.gov
DRUG FACTS Ocp Prescription Label Requirements Office of communications division of drug information center for drug evaluation and research food and drug administration 10903. (a) no drug may be dispensed by outpatient prescription unless a. The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or. A product comprised of two or more regulated components (i.e., drug/device, biologic/device,. Ocp Prescription Label Requirements.