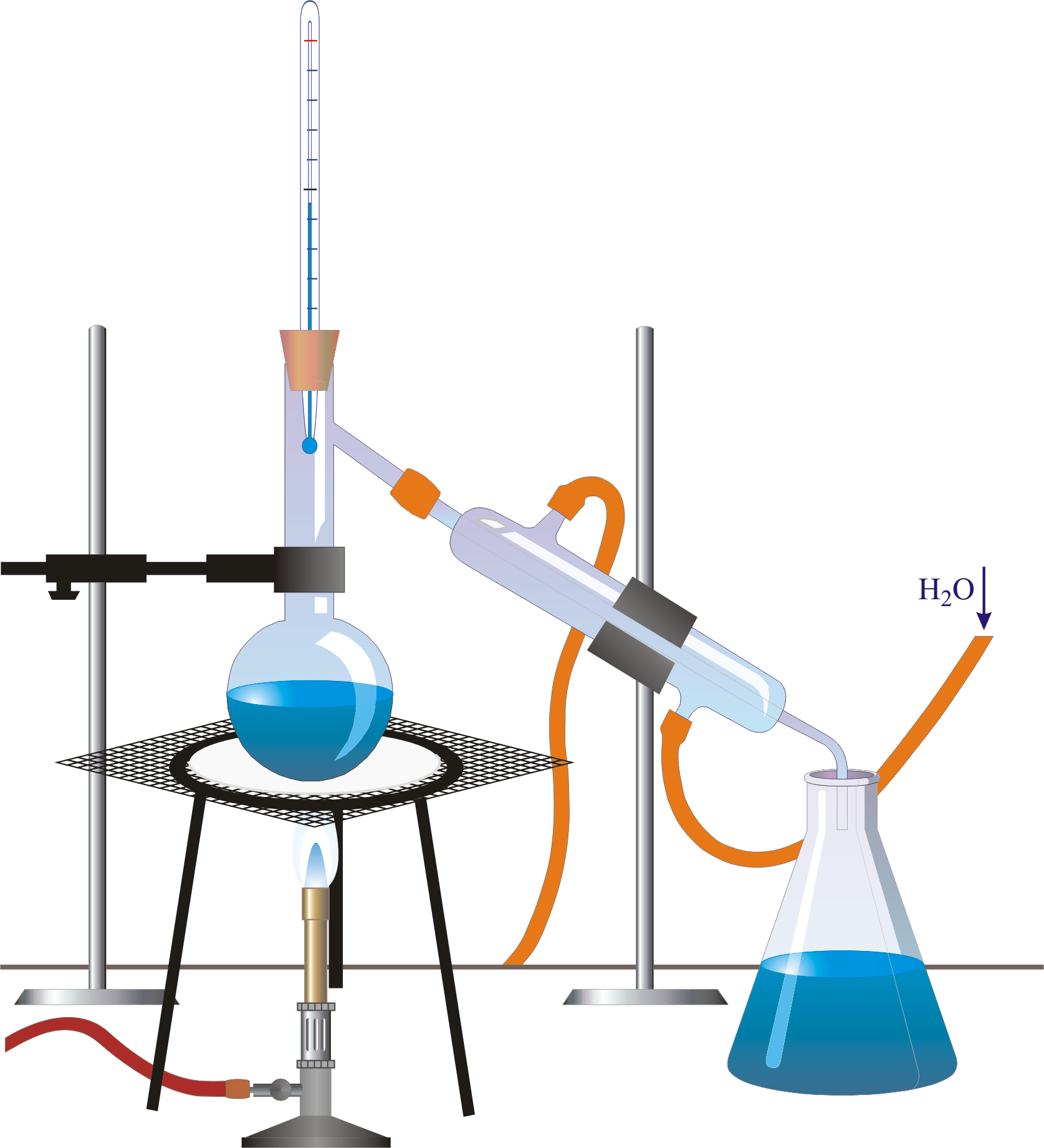
Purpose Of Distillation In Esterification . The chemistry of the reaction. The chemistry of the reaction. The esterification reaction is both slow and reversible. Making esters from carboxylic acids and alcohols. The equation for the reaction between an acid rcooh and an alcohol r'oh. Than the ester and is easily removed by simple distillation. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The ether has a much lower b.p. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Esterification of acetic acid and isopentyl alcohol (figure 3). Isolate the crude ester by simple distillation. Synthesis or preparation of esters in the. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Heat under reflux to prepare the ester. The reaction will be catalyzed by the addition of sulfuric acid.
from glossary.periodni.com
The equation for the reaction between an acid rcooh and an alcohol r'oh. Making esters from carboxylic acids and alcohols. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. The esterification reaction is both slow and reversible. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. Heat under reflux to prepare the ester. Synthesis or preparation of esters in the. Esterification of acetic acid and isopentyl alcohol (figure 3).
Distillation Chemistry Dictionary & Glossary
Purpose Of Distillation In Esterification The equation for the reaction between an acid rcooh and an alcohol r'oh. The reaction will be catalyzed by the addition of sulfuric acid. Than the ester and is easily removed by simple distillation. Esterification of acetic acid and isopentyl alcohol (figure 3). Synthesis or preparation of esters in the. Making esters from carboxylic acids and alcohols. The chemistry of the reaction. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The ether has a much lower b.p. The equation for the reaction between an acid rcooh and an alcohol r'oh. The chemistry of the reaction. Isolate the crude ester by simple distillation. Heat under reflux to prepare the ester. The esterification reaction is both slow and reversible. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of.
From pharmaguides.in
3.4 Differences Between Distillation And Fractional Distillation Purpose Of Distillation In Esterification Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The ether has a much lower b.p. Esterification of acetic acid and isopentyl alcohol (figure 3). The chemistry of the reaction. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. Isolate the crude ester by simple distillation.. Purpose Of Distillation In Esterification.
From www.scienceabc.com
Denatured Alcohol Definition, Properties, Examples And Uses Purpose Of Distillation In Esterification Heat under reflux to prepare the ester. Than the ester and is easily removed by simple distillation. The chemistry of the reaction. The equation for the reaction between an acid rcooh and an alcohol r'oh. Making esters from carboxylic acids and alcohols. Synthesis or preparation of esters in the. The ether has a much lower b.p. Phenols react with carboxylic. Purpose Of Distillation In Esterification.
From chemsept.blogspot.com
DISTILLATION COLUMN HISTORY Purpose Of Distillation In Esterification Synthesis or preparation of esters in the. The chemistry of the reaction. Heat under reflux to prepare the ester. Making esters from carboxylic acids and alcohols. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The esterification reaction is both slow and reversible. Esterification of acetic acid and isopentyl alcohol (figure 3). The ether. Purpose Of Distillation In Esterification.
From www.researchgate.net
(PDF) Performance of esterification system in reactiondistillation column Purpose Of Distillation In Esterification Isolate the crude ester by simple distillation. The chemistry of the reaction. The esterification reaction is both slow and reversible. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The equation for the reaction between an acid rcooh and an alcohol r'oh. The chemistry of the reaction. Synthesis or preparation of esters in. Purpose Of Distillation In Esterification.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Purpose Of Distillation In Esterification The chemistry of the reaction. Synthesis or preparation of esters in the. The esterification reaction is both slow and reversible. The equation for the reaction between an acid rcooh and an alcohol r'oh. Esterification of acetic acid and isopentyl alcohol (figure 3). The ether has a much lower b.p. Heat under reflux to prepare the ester. The chemistry of the. Purpose Of Distillation In Esterification.
From www.slideserve.com
PPT Chapter 40 Esterification. Synthesis of n Butyl Acetate by Purpose Of Distillation In Esterification To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. The equation for the reaction between an acid rcooh and an alcohol r'oh. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Esters are produced when carboxylic acids are heated with. Purpose Of Distillation In Esterification.
From docslib.org
Isolation and Purification of Organic Compounds Steam Distillation Of Purpose Of Distillation In Esterification Synthesis or preparation of esters in the. Heat under reflux to prepare the ester. The ether has a much lower b.p. The equation for the reaction between an acid rcooh and an alcohol r'oh. The chemistry of the reaction. Isolate the crude ester by simple distillation. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation. Purpose Of Distillation In Esterification.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Purpose Of Distillation In Esterification Isolate the crude ester by simple distillation. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Heat under reflux to prepare the ester. The esterification reaction is both slow and reversible. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of.. Purpose Of Distillation In Esterification.
From www.youtube.com
Esterification Reflux, Isolation and Purification // HSC Chemistry Purpose Of Distillation In Esterification Isolate the crude ester by simple distillation. The esterification reaction is both slow and reversible. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. The reaction will be catalyzed by the addition of sulfuric acid. The equation for the reaction between an acid rcooh and an. Purpose Of Distillation In Esterification.
From www.solutionspile.com
[Solved] The figure above illustrates a distillation column Purpose Of Distillation In Esterification The equation for the reaction between an acid rcooh and an alcohol r'oh. Heat under reflux to prepare the ester. The chemistry of the reaction. The ether has a much lower b.p. The reaction will be catalyzed by the addition of sulfuric acid. Making esters from carboxylic acids and alcohols. Phenols react with carboxylic acids so slowly that the reaction. Purpose Of Distillation In Esterification.
From pharmaguides.in
Distillation Definition Why Is The Process Of Fractional Distillation Purpose Of Distillation In Esterification Heat under reflux to prepare the ester. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. Esterification of acetic acid and isopentyl alcohol (figure 3). To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Phenols react with carboxylic acids. Purpose Of Distillation In Esterification.
From www.slideserve.com
PPT Chapter 40 Esterification. Synthesis of n Butyl Acetate by Purpose Of Distillation In Esterification Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The chemistry of the reaction. The chemistry of the reaction. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. The esterification reaction is both slow and reversible. Heat under reflux to. Purpose Of Distillation In Esterification.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps Purpose Of Distillation In Esterification Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The equation for the reaction between an acid rcooh and an alcohol r'oh. Than the ester and is easily removed by simple distillation. The reaction will be catalyzed by the addition of sulfuric acid. The ether has a much lower b.p. Isolate the crude. Purpose Of Distillation In Esterification.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Purpose Of Distillation In Esterification The chemistry of the reaction. Esterification of acetic acid and isopentyl alcohol (figure 3). The chemistry of the reaction. Isolate the crude ester by simple distillation. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Phenols react with carboxylic acids so slowly that the reaction is. Purpose Of Distillation In Esterification.
From jupiter.plymouth.edu
DISTILLATION APPARATUS Purpose Of Distillation In Esterification Synthesis or preparation of esters in the. The chemistry of the reaction. Esterification of acetic acid and isopentyl alcohol (figure 3). Isolate the crude ester by simple distillation. Than the ester and is easily removed by simple distillation. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The ether has a much lower. Purpose Of Distillation In Esterification.
From www.chegg.com
Solved There is supposed to be a chemical reaction for this Purpose Of Distillation In Esterification Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. Heat under reflux to prepare the ester. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. Isolate the crude ester by simple. Purpose Of Distillation In Esterification.
From www.youtube.com
Distillation Definition and Technique Basic Principles and Purpose Of Distillation In Esterification Esterification of acetic acid and isopentyl alcohol (figure 3). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The equation for the reaction between an acid rcooh and an alcohol r'oh. Heat under reflux to prepare the ester. The reaction will be catalyzed by the addition of sulfuric acid. Phenols react with carboxylic. Purpose Of Distillation In Esterification.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Purpose Of Distillation In Esterification Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Making esters from carboxylic acids and alcohols. The esterification reaction is both slow and reversible. Esterification of acetic acid and isopentyl alcohol (figure 3). The chemistry of the reaction. Heat under reflux to prepare the ester. Esters are produced when carboxylic acids are heated with. Purpose Of Distillation In Esterification.
From www.slideserve.com
PPT Chapter 40 Esterification. Synthesis of n Butyl Acetate by Purpose Of Distillation In Esterification The chemistry of the reaction. The ether has a much lower b.p. The reaction will be catalyzed by the addition of sulfuric acid. Making esters from carboxylic acids and alcohols. Than the ester and is easily removed by simple distillation. The esterification reaction is both slow and reversible. Heat under reflux to prepare the ester. Isolate the crude ester by. Purpose Of Distillation In Esterification.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Purpose Of Distillation In Esterification Esterification of acetic acid and isopentyl alcohol (figure 3). Synthesis or preparation of esters in the. The reaction will be catalyzed by the addition of sulfuric acid. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Phenols react with carboxylic acids so slowly that the reaction. Purpose Of Distillation In Esterification.
From www.askiitians.com
Organic Chemistry Purification of organic compounds askIITians Purpose Of Distillation In Esterification Making esters from carboxylic acids and alcohols. Esterification of acetic acid and isopentyl alcohol (figure 3). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Than the ester and is easily removed by simple distillation. The equation for. Purpose Of Distillation In Esterification.
From www.slideserve.com
PPT Esterification PowerPoint Presentation, free download ID3150303 Purpose Of Distillation In Esterification The equation for the reaction between an acid rcooh and an alcohol r'oh. The ether has a much lower b.p. Than the ester and is easily removed by simple distillation. Making esters from carboxylic acids and alcohols. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of.. Purpose Of Distillation In Esterification.
From glossary.periodni.com
Distillation Chemistry Dictionary & Glossary Purpose Of Distillation In Esterification Esterification of acetic acid and isopentyl alcohol (figure 3). Isolate the crude ester by simple distillation. Heat under reflux to prepare the ester. The chemistry of the reaction. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The reaction will be catalyzed by the addition of sulfuric acid. Esters are produced when carboxylic acids. Purpose Of Distillation In Esterification.
From medium.com
‘Ultrapure Water and Distilled Water — What is the Difference? by Purpose Of Distillation In Esterification Isolate the crude ester by simple distillation. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. Than the ester and is easily removed by simple distillation. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. Esterification of acetic acid and isopentyl alcohol (figure 3). The ether. Purpose Of Distillation In Esterification.
From www.youtube.com
Chemistry A Level practical techniques reflux and distillation YouTube Purpose Of Distillation In Esterification Than the ester and is easily removed by simple distillation. Heat under reflux to prepare the ester. Making esters from carboxylic acids and alcohols. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The esterification reaction is both slow and reversible. The equation for the reaction between an acid rcooh and an alcohol. Purpose Of Distillation In Esterification.
From brainly.in
Explain the process of simple distillation with diagram Brainly.in Purpose Of Distillation In Esterification The ether has a much lower b.p. Making esters from carboxylic acids and alcohols. Isolate the crude ester by simple distillation. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Esterification of acetic acid and isopentyl alcohol (figure 3). The chemistry of the reaction. Than the. Purpose Of Distillation In Esterification.
From www.researchgate.net
[PDF] Reactive Distillation for Esterification Reaction of Isoamyl Purpose Of Distillation In Esterification Isolate the crude ester by simple distillation. Than the ester and is easily removed by simple distillation. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The equation for the reaction between an acid rcooh and an alcohol r'oh. To make a small ester like ethyl ethanoate, you can gently heat a mixture. Purpose Of Distillation In Esterification.
From www.animalia-life.club
Esterification Mechanism Purpose Of Distillation In Esterification The esterification reaction is both slow and reversible. The chemistry of the reaction. Isolate the crude ester by simple distillation. To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Making esters from carboxylic acids and alcohols. Esters are produced when carboxylic acids are heated with alcohols. Purpose Of Distillation In Esterification.
From www.researchgate.net
(PDF) Esterification of Acetic Acid with Butanol Operation in a Packed Purpose Of Distillation In Esterification Than the ester and is easily removed by simple distillation. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The chemistry of the reaction. The esterification reaction is both slow and reversible. Synthesis or preparation of esters in the. The equation for the reaction between an acid rcooh and an alcohol r'oh. The ether. Purpose Of Distillation In Esterification.
From www.vrogue.co
The Various Types Of Distillation That Are Worth Know vrogue.co Purpose Of Distillation In Esterification Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. The ether has a much lower b.p. Isolate the crude ester by simple distillation. Making esters from carboxylic acids and alcohols. The equation for the reaction between an acid rcooh and an alcohol r'oh. To make a small ester like ethyl ethanoate, you can gently. Purpose Of Distillation In Esterification.
From www.semanticscholar.org
[PDF] Catalytic Reactive Distillation for the Esterification Process Purpose Of Distillation In Esterification To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Heat under reflux to prepare the ester. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid. The chemistry of the reaction. Isolate the crude ester by simple distillation. The ether. Purpose Of Distillation In Esterification.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Purpose Of Distillation In Esterification Synthesis or preparation of esters in the. Making esters from carboxylic acids and alcohols. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. The ether has a much lower b.p. Than the ester and is easily removed by simple distillation. Heat under reflux to prepare the ester. Phenols react with carboxylic acids so. Purpose Of Distillation In Esterification.
From testbook.com
team Distillation Principle, Working, Advantages & Application Purpose Of Distillation In Esterification To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. Heat under reflux to prepare the ester. The chemistry of the reaction. The reaction will be catalyzed by the addition of sulfuric acid. Than the ester and is easily removed by simple distillation. Phenols react with carboxylic. Purpose Of Distillation In Esterification.
From www.chegg.com
Solved 7. What is the purpose of Distillation? 8. What does Purpose Of Distillation In Esterification To make a small ester like ethyl ethanoate, you can gently heat a mixture of ethanoic acid and ethanol in the presence of. The reaction will be catalyzed by the addition of sulfuric acid. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Esterification of acetic acid and isopentyl alcohol (figure 3). The chemistry. Purpose Of Distillation In Esterification.
From henanlanphan.en.made-in-china.com
Dual Multi Stages Green Distiller Molecular Distillation Esterification Purpose Of Distillation In Esterification The reaction will be catalyzed by the addition of sulfuric acid. Heat under reflux to prepare the ester. Phenols react with carboxylic acids so slowly that the reaction is unusable for preparation purposes. Synthesis or preparation of esters in the. The ether has a much lower b.p. Isolate the crude ester by simple distillation. Making esters from carboxylic acids and. Purpose Of Distillation In Esterification.