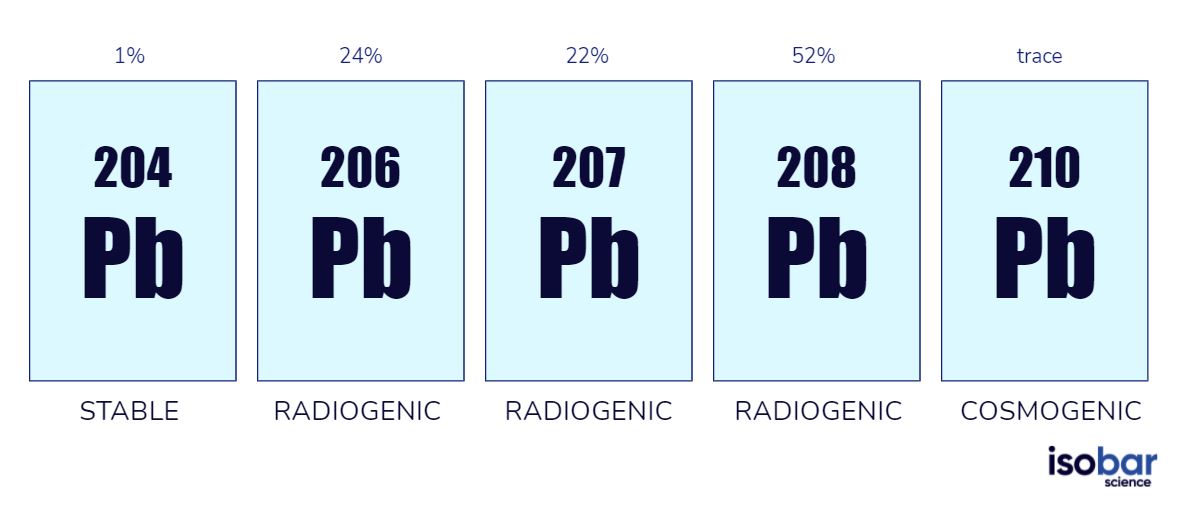
Isotopes Of Lead Atomic Mass . The total number of neutrons in the nucleus of an atom is called the. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. These consist of an atomic nucleus with. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 119 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. mass number of lead. all atomic nuclei of the chemical element lead are summarized under lead isotopes;
from isobarscience.com
119 rows lead (82 pb) has four observationally stable isotopes: Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. mass number of lead. all atomic nuclei of the chemical element lead are summarized under lead isotopes; The total number of neutrons in the nucleus of an atom is called the. the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. These consist of an atomic nucleus with. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic.
Lead Isotopes Geochemistry Isobar Science
Isotopes Of Lead Atomic Mass mass number of lead. the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical. all atomic nuclei of the chemical element lead are summarized under lead isotopes; mass number of lead. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. The total number of neutrons in the nucleus of an atom is called the. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. 119 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. These consist of an atomic nucleus with.
From ar.inspiredpencil.com
Lead Atomic Mass Isotopes Of Lead Atomic Mass mass number of lead. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. The total number of neutrons in the nucleus of an atom is called the. 204 pb, 206 pb, 207 pb, 208 pb. Sources, facts, uses, scarcity (sri), podcasts, alchemical. the isotopes of an. Isotopes Of Lead Atomic Mass.
From shaunmwilliams.com
Lecture 15 Presentation Isotopes Of Lead Atomic Mass 204 pb, 206 pb, 207 pb, 208 pb. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. The total number of neutrons in the nucleus of an atom is called the. the isotopes of an element differ only in their atomic mass, which is given by the. Isotopes Of Lead Atomic Mass.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Isotopes Of Lead Atomic Mass These consist of an atomic nucleus with. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. The total number of neutrons in the nucleus of an atom is called the. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. Sources,. Isotopes Of Lead Atomic Mass.
From www.slideserve.com
PPT The Atom Atomic Number Mass Number Isotopes PowerPoint Presentation ID6473854 Isotopes Of Lead Atomic Mass atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. 204 pb, 206 pb, 207 pb, 208 pb. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. mass number of lead. The total number of neutrons in the nucleus of. Isotopes Of Lead Atomic Mass.
From material-properties.org
Lead Periodic Table and Atomic Properties Isotopes Of Lead Atomic Mass this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. The total number of neutrons in the nucleus of an atom is called the. 204 pb, 206. Isotopes Of Lead Atomic Mass.
From exohwyylp.blob.core.windows.net
Atomic Mass Of Lead Chloride at Vincent Buck blog Isotopes Of Lead Atomic Mass the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. 204 pb, 206 pb, 207 pb, 208 pb. mass number of lead. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. Sources, facts,. Isotopes Of Lead Atomic Mass.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry Isotopes Of Lead Atomic Mass Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. These consist of an atomic nucleus with. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. 119 rows lead (82 pb) has four observationally stable. Isotopes Of Lead Atomic Mass.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry Isotopes Of Lead Atomic Mass Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. 204 pb, 206 pb, 207 pb, 208 pb. all atomic nuclei of the chemical element lead are summarized under lead isotopes; mass number of lead. These consist of an atomic nucleus with. the isotopes of. Isotopes Of Lead Atomic Mass.
From slideplayer.com
Midterm Review Chemistry. ppt download Isotopes Of Lead Atomic Mass These consist of an atomic nucleus with. the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. 204 pb, 206 pb, 207 pb, 208 pb. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic.. Isotopes Of Lead Atomic Mass.
From www.sciencephoto.com
Lead, atomic structure Stock Image C018/3763 Science Photo Library Isotopes Of Lead Atomic Mass 204 pb, 206 pb, 207 pb, 208 pb. These consist of an atomic nucleus with. 119 rows lead (82 pb) has four observationally stable isotopes: this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. all atomic nuclei of the chemical element lead are summarized under lead. Isotopes Of Lead Atomic Mass.
From isobarscience.com
Lead Isotopes Geochemistry Isobar Science Isotopes Of Lead Atomic Mass The total number of neutrons in the nucleus of an atom is called the. all atomic nuclei of the chemical element lead are summarized under lead isotopes; atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their. Isotopes Of Lead Atomic Mass.
From www.centraldatacore.com
082 Lead Pb Isotopes Of Lead Atomic Mass mass number of lead. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. 204 pb, 206 pb, 207 pb, 208 pb. 119 rows lead (82 pb) has four observationally stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical. this table shows information about naturally. Isotopes Of Lead Atomic Mass.
From slidetodoc.com
Calculating the Average Atomic Mass Number Where did Isotopes Of Lead Atomic Mass the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. 204 pb, 206 pb, 207 pb, 208 pb. The total number of neutrons in the nucleus of an atom is called the. all atomic nuclei of the chemical element lead. Isotopes Of Lead Atomic Mass.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Chemistry 103/104 Resource Book Isotopes Of Lead Atomic Mass The total number of neutrons in the nucleus of an atom is called the. mass number of lead. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. Sources, facts, uses,. Isotopes Of Lead Atomic Mass.
From www.researchgate.net
Proposed element cell for lead for the IUPAC Periodic Table of the... Download Scientific Diagram Isotopes Of Lead Atomic Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical. 204 pb, 206 pb, 207 pb, 208 pb. 119 rows lead (82 pb) has four observationally stable isotopes: atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. The total number of neutrons in the nucleus of an atom is called the. mass number of. Isotopes Of Lead Atomic Mass.
From www.chegg.com
Solved The four isotopes of lead are shown below. * Isotopes Of Lead Atomic Mass The total number of neutrons in the nucleus of an atom is called the. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. mass number of lead. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and. Isotopes Of Lead Atomic Mass.
From www.transtutors.com
(Get Answer) The Four Isotopes Of Lead Are Shown Below, Each With Its Percent... Transtutors Isotopes Of Lead Atomic Mass mass number of lead. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. all atomic nuclei of the chemical element lead are summarized under lead isotopes; The total number of neutrons in the nucleus of an atom is. Isotopes Of Lead Atomic Mass.
From maimelatct.com
ISOTOPE AND RELATIVE ATOMIC MASS Physical sciences break 1.0 Isotopes Of Lead Atomic Mass mass number of lead. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. 204 pb, 206 pb, 207 pb, 208 pb. all atomic nuclei of the chemical element lead are summarized under lead isotopes; 119 rows lead (82 pb) has four observationally stable isotopes: the isotopes of an element. Isotopes Of Lead Atomic Mass.
From www.slideserve.com
PPT Internal Structure of Atoms PowerPoint Presentation, free download ID9683214 Isotopes Of Lead Atomic Mass atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. mass number of lead. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. 119 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb,. Isotopes Of Lead Atomic Mass.
From www.numerade.com
SOLVED Lead, chemical symbol Pb, has atomic number 82 and can form Pb2+ and Pb4+ ions. This Isotopes Of Lead Atomic Mass atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. all atomic nuclei of the chemical element lead are summarized under lead isotopes; These consist of an atomic nucleus with. The total number of neutrons in the nucleus of an atom is called the. Sources, facts, uses, scarcity (sri), podcasts, alchemical. the. Isotopes Of Lead Atomic Mass.
From murov.info
ATOMIC MASS and ISOTOPES Isotopes Of Lead Atomic Mass mass number of lead. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. 119 rows lead (82 pb) has four observationally stable isotopes: this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. These. Isotopes Of Lead Atomic Mass.
From general.chemistrysteps.com
What Are Isotopes? Chemistry Steps Isotopes Of Lead Atomic Mass atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. The total number of neutrons in the nucleus of an atom is called the. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. mass number of lead. this table. Isotopes Of Lead Atomic Mass.
From www.slideserve.com
PPT Dating with Isotopes PowerPoint Presentation, free download ID227553 Isotopes Of Lead Atomic Mass 119 rows lead (82 pb) has four observationally stable isotopes: These consist of an atomic nucleus with. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. Sources, facts, uses, scarcity (sri), podcasts, alchemical. mass number of lead. the isotopes of an element differ only. Isotopes Of Lead Atomic Mass.
From www.slideserve.com
PPT Isotopes, Atomic Numbers, and Mass Numbers PowerPoint Presentation ID6700609 Isotopes Of Lead Atomic Mass These consist of an atomic nucleus with. Sources, facts, uses, scarcity (sri), podcasts, alchemical. all atomic nuclei of the chemical element lead are summarized under lead isotopes; 119 rows lead (82 pb) has four observationally stable isotopes: the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the. Isotopes Of Lead Atomic Mass.
From brainly.com
Calculate the atomic mass of lead. The four lead isotopes have atomic masses and relative Isotopes Of Lead Atomic Mass 119 rows lead (82 pb) has four observationally stable isotopes: this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. all atomic nuclei of the chemical element lead are summarized under lead isotopes; Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207,. Isotopes Of Lead Atomic Mass.
From www.numerade.com
SOLVED Four Isotopes Of lead are shown below; each wlth Its percent by mass abundance and mass Isotopes Of Lead Atomic Mass the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances,. Isotopes Of Lead Atomic Mass.
From www.slideserve.com
PPT Nuclear Chemistry PowerPoint Presentation, free download ID6876282 Isotopes Of Lead Atomic Mass These consist of an atomic nucleus with. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 204 pb, 206 pb, 207 pb, 208 pb. The total number of neutrons in the nucleus of an atom is called the. the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the. Isotopes Of Lead Atomic Mass.
From ar.inspiredpencil.com
Lead Atomic Number Isotopes Of Lead Atomic Mass The total number of neutrons in the nucleus of an atom is called the. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. all atomic nuclei of the chemical element lead are summarized under lead isotopes; this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their. Isotopes Of Lead Atomic Mass.
From www.youtube.com
How to Calculate the Average Atomic Mass of Lead YouTube Isotopes Of Lead Atomic Mass 204 pb, 206 pb, 207 pb, 208 pb. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. 119 rows lead (82 pb) has four observationally stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical. The total number of neutrons in the nucleus of an atom is called. Isotopes Of Lead Atomic Mass.
From www.istockphoto.com
10+ Electron Configuration Lead Stock Photos, Pictures & RoyaltyFree Images iStock Isotopes Of Lead Atomic Mass all atomic nuclei of the chemical element lead are summarized under lead isotopes; the isotopes of an element differ only in their atomic mass, which is given by the mass number (a), the sum of the numbers of protons and neutrons. These consist of an atomic nucleus with. mass number of lead. Natural lead consists of four. Isotopes Of Lead Atomic Mass.
From quizlet.com
Isotopes of lead Diagram Quizlet Isotopes Of Lead Atomic Mass 119 rows lead (82 pb) has four observationally stable isotopes: The total number of neutrons in the nucleus of an atom is called the. These consist of an atomic nucleus with. all atomic nuclei of the chemical element lead are summarized under lead isotopes; Sources, facts, uses, scarcity (sri), podcasts, alchemical. this table shows information about naturally. Isotopes Of Lead Atomic Mass.
From www.youtube.com
Atomic number, mass number, and isotopes Chemistry Khan Academy YouTube Isotopes Of Lead Atomic Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical. 204 pb, 206 pb, 207 pb, 208 pb. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. These consist of an atomic nucleus with. The total number of neutrons in the nucleus of an atom is called the. the. Isotopes Of Lead Atomic Mass.
From www.tes.com
Isotopes and Relative Atomic Mass GCSE Lesson (SC3c CC3c) Teaching Resources Isotopes Of Lead Atomic Mass 204 pb, 206 pb, 207 pb, 208 pb. The total number of neutrons in the nucleus of an atom is called the. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. 119 rows lead (82 pb) has four observationally stable isotopes: Natural lead consists of four stable isotopes with mass numbers of. Isotopes Of Lead Atomic Mass.
From www.numerade.com
SOLVED The four isotopes of lead are shown below, each with its percent by mass abundance, the Isotopes Of Lead Atomic Mass Sources, facts, uses, scarcity (sri), podcasts, alchemical. Natural lead consists of four stable isotopes with mass numbers of 204, 206, 207, and 208, [37] and traces of six short. 204 pb, 206 pb, 207 pb, 208 pb. atomic weights and isotopic compositions for lead isotope relative atomic mass isotopic composition standard atomic. mass number of lead. These consist. Isotopes Of Lead Atomic Mass.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Isotopes Of Lead Atomic Mass The total number of neutrons in the nucleus of an atom is called the. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 119 rows lead (82 pb) has four observationally stable isotopes: all atomic nuclei of the chemical element lead are summarized under lead isotopes; mass number of lead. 204 pb, 206 pb, 207 pb, 208 pb. Natural. Isotopes Of Lead Atomic Mass.