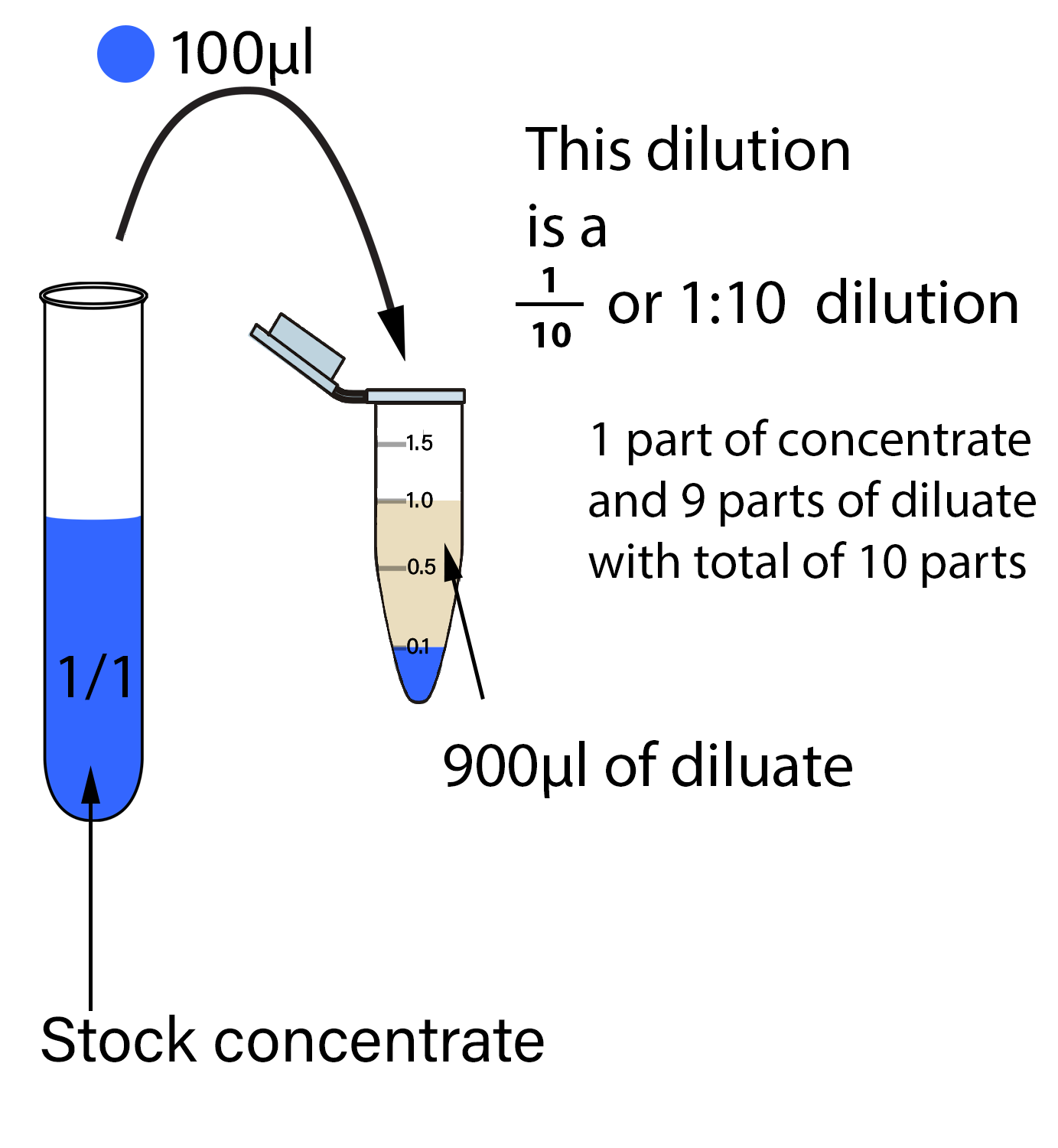
How To Do A 100 Fold Dilution . Generate a standard curve and. Dilution is the process of making a solution weaker or less concentrated. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. Use the spectrophotometer to measure the absorbance of solutions. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required.
from www.medicine.mcgill.ca
In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. Generate a standard curve and. Use the spectrophotometer to measure the absorbance of solutions. Dilution is the process of making a solution weaker or less concentrated. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution?
Serial Dilutions
How To Do A 100 Fold Dilution In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Dilution is the process of making a solution weaker or less concentrated. Generate a standard curve and. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. Use the spectrophotometer to measure the absorbance of solutions. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of solutions. Dilution is the process of making a solution weaker or. How To Do A 100 Fold Dilution.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate How To Do A 100 Fold Dilution Generate a standard curve and. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about. How To Do A 100 Fold Dilution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. Generate a standard curve and. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In the. How To Do A 100 Fold Dilution.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. Generate a standard curve and. In microbiology, serial dilutions (log dilutions) are used. How To Do A 100 Fold Dilution.
From www.medicine.mcgill.ca
Serial Dilutions How To Do A 100 Fold Dilution In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of solutions. Dilution is the process. How To Do A 100 Fold Dilution.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. Use the spectrophotometer to measure the absorbance of solutions. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2. How To Do A 100 Fold Dilution.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Dilution is the process of making a solution weaker or less concentrated. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. Generate a standard curve and. For example, if 100 ml of a stock solution is. How To Do A 100 Fold Dilution.
From www.thetechedvocate.org
How to Calculate Serial Dilutions The Tech Edvocate How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? The first tube will be a 1:10 dilution, the second a 1:100,. How To Do A 100 Fold Dilution.
From study.com
Serial Dilution in Microbiology Calculation, Method & Technique How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Generate a standard curve and. Dilution is the process of making a solution weaker or less concentrated. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The formula. How To Do A 100 Fold Dilution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Do A 100 Fold Dilution Use the spectrophotometer to measure the absorbance of solutions. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Dilution is the process of making a solution weaker or less. How To Do A 100 Fold Dilution.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. Generate a standard curve and. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Use the spectrophotometer to measure the absorbance of solutions. For. How To Do A 100 Fold Dilution.
From mungfali.com
10 Fold Serial Dilution How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution is the process of making a solution weaker or less concentrated. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Use the spectrophotometer to measure the absorbance. How To Do A 100 Fold Dilution.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The. How To Do A 100 Fold Dilution.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA How To Do A 100 Fold Dilution Generate a standard curve and. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to. How To Do A 100 Fold Dilution.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow How To Do A 100 Fold Dilution Generate a standard curve and. Use the spectrophotometer to measure the absorbance of solutions. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. Dilution is the process of making a solution weaker or less concentrated. For example, if 100 ml of a stock solution is diluted. How To Do A 100 Fold Dilution.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Use the spectrophotometer to measure the absorbance of solutions. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. In the first example above, we wanted to dilute a sample. How To Do A 100 Fold Dilution.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed. How To Do A 100 Fold Dilution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew How To Do A 100 Fold Dilution In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. Generate a standard curve and. Use the spectrophotometer to measure the absorbance of solutions. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? The first tube will be a. How To Do A 100 Fold Dilution.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY How To Do A 100 Fold Dilution Generate a standard curve and. Use the spectrophotometer to measure the absorbance of solutions. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The formula for serial dilutions is df = df_1 × df_2 × df_3. How To Do A 100 Fold Dilution.
From www.youtube.com
U10L4 Molarity, Dilution, PPM, and Molality Calculations YouTube How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Use the spectrophotometer. How To Do A 100 Fold Dilution.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of solutions. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. How To Do A 100 Fold Dilution.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria How To Do A 100 Fold Dilution In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Use the spectrophotometer to measure the absorbance of solutions. Dilution is the process of making a solution weaker or less. How To Do A 100 Fold Dilution.
From www.numerade.com
SOLVED How would you perform a 100fold dilution, if you want the How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of solutions. Generate a standard. How To Do A 100 Fold Dilution.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution. How To Do A 100 Fold Dilution.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY How To Do A 100 Fold Dilution The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Use the spectrophotometer to measure the absorbance of solutions. Generate a standard. How To Do A 100 Fold Dilution.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Generate a standard curve and. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of solutions. In the first example above,. How To Do A 100 Fold Dilution.
From bdaschools.weebly.com
bdaschools Blog How To Do A 100 Fold Dilution The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Generate a standard curve and. Use the spectrophotometer to measure the absorbance of solutions. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. In microbiology, serial dilutions (log. How To Do A 100 Fold Dilution.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How To Do A 100 Fold Dilution The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Generate a standard curve and. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution is the process of making a. How To Do A 100 Fold Dilution.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the spectrophotometer to measure the absorbance of. How To Do A 100 Fold Dilution.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples How To Do A 100 Fold Dilution The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Dilution is the process of making a solution weaker or less concentrated. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? In microbiology, serial dilutions (log dilutions) are. How To Do A 100 Fold Dilution.
From www.chegg.com
Solved 100fold dilution (1/100 dilution) of a 150 LM How To Do A 100 Fold Dilution For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution is the process of making a solution weaker or less concentrated. Use the spectrophotometer to measure the absorbance of solutions. In the first example above, we wanted to dilute a sample with an incredibly high. How To Do A 100 Fold Dilution.
From www.youtube.com
AS Biology How to calculate serial and simple dilutions YouTube How To Do A 100 Fold Dilution Dilution is the process of making a solution weaker or less concentrated. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Generate a standard curve and. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? Use the. How To Do A 100 Fold Dilution.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples How To Do A 100 Fold Dilution In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily. How To Do A 100 Fold Dilution.
From www.chegg.com
Solved 100fold dilution (1/100 dilution) of a 150 LM How To Do A 100 Fold Dilution Generate a standard curve and. The formula for serial dilutions is df = df_1 × df_2 × df_3 ×… how about a 1:100 dilution followed by a 1:2 dilution? The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Dilution is the process of making a solution weaker or less concentrated. In the. How To Do A 100 Fold Dilution.
From userpages.umbc.edu
DILUTE How To Do A 100 Fold Dilution Use the spectrophotometer to measure the absorbance of solutions. In the first example above, we wanted to dilute a sample with an incredibly high bacterial concentration to an easily countable number of. Generate a standard curve and. Dilution is the process of making a solution weaker or less concentrated. The formula for serial dilutions is df = df_1 × df_2. How To Do A 100 Fold Dilution.