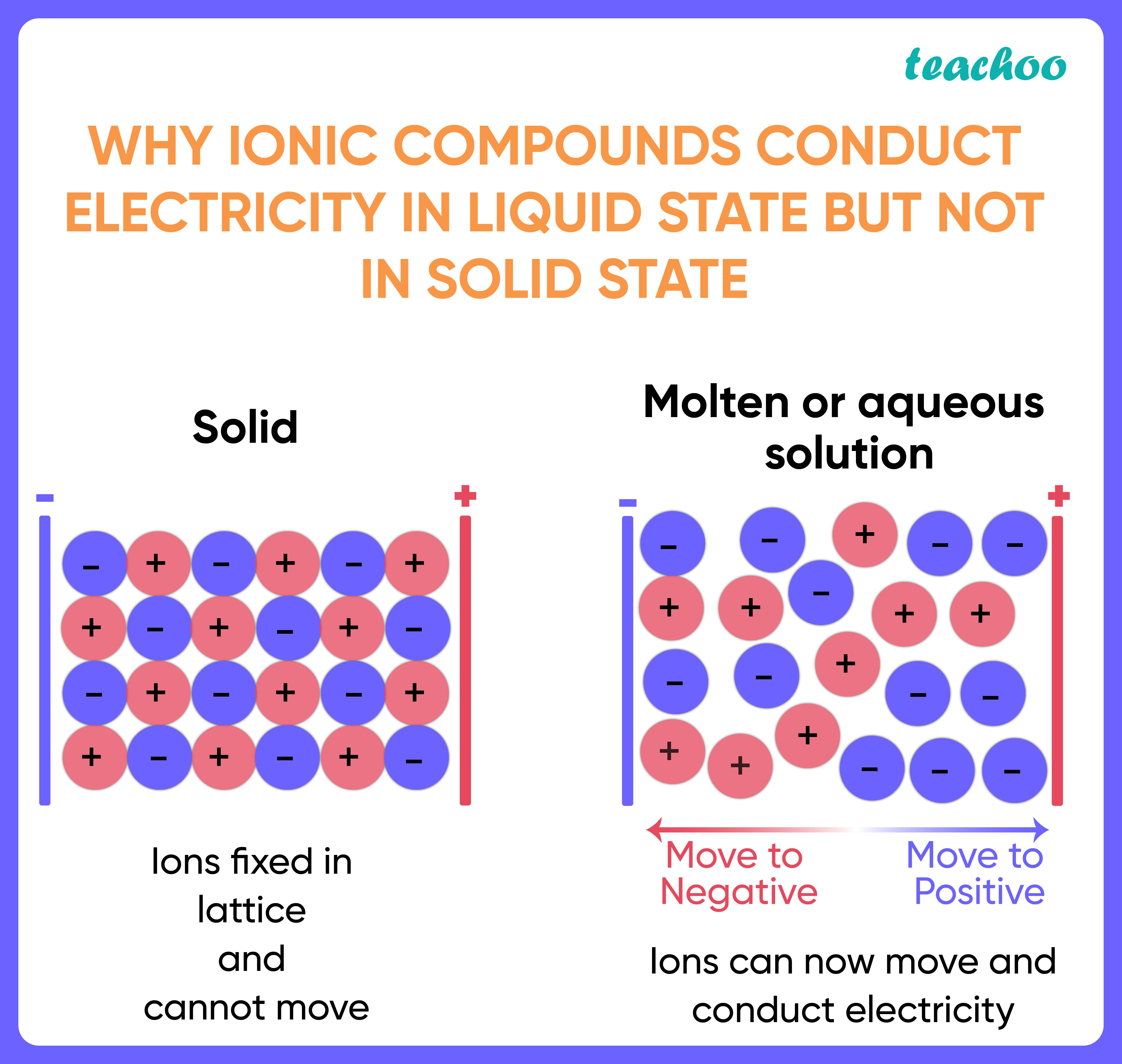
Why Are Ionic Compounds Good Conductors Of Heat . Will melted ionic compounds conduct electricity? In an ionic solution, the a + ions. However, they do conduct when molten or dissolved because their ions are free to. What are the melting and boiling points of ki? do all ionic compounds form crystals? high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic compounds also conduct heat as the ions jiggle in their positions. once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. ionic solids do not conduct electricity; ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds tend to be crystalline structures with high melting points that are water soluble.
from www.teachoo.com
However, they do conduct when molten or dissolved because their ions are free to. ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. ionic compounds tend to be crystalline structures with high melting points that are water soluble. do all ionic compounds form crystals? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic solids do not conduct electricity; Will melted ionic compounds conduct electricity? ionic compounds also conduct heat as the ions jiggle in their positions. What are the melting and boiling points of ki?
The table shown below gives information about four Chemistry MCQ
Why Are Ionic Compounds Good Conductors Of Heat do all ionic compounds form crystals? Will melted ionic compounds conduct electricity? In an ionic solution, the a + ions. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. What are the melting and boiling points of ki? ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds tend to be crystalline structures with high melting points that are water soluble. However, they do conduct when molten or dissolved because their ions are free to. ionic solids do not conduct electricity; once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. do all ionic compounds form crystals? ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through.
From seanrtmckee.blogspot.com
Good Conductor of Heat SeanrtMckee Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. do all ionic compounds form crystals? What are the melting and. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideshare.net
Chapter 24 Conduction Why Are Ionic Compounds Good Conductors Of Heat In an ionic solution, the a + ions. However, they do conduct when molten or dissolved because their ions are free to. ionic compounds conduct an electric current when melted or dissolved in water. do all ionic compounds form crystals? ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. What. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Unit 6 Ionic and Covalent Bonding ppt download Why Are Ionic Compounds Good Conductors Of Heat What are the melting and boiling points of ki? ionic compounds also conduct heat as the ions jiggle in their positions. ionic solids do not conduct electricity; ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. ionic compounds tend to be crystalline structures with high melting points that are. Why Are Ionic Compounds Good Conductors Of Heat.
From dxowfrleu.blob.core.windows.net
Good Conductors Thermal Energy at Cecil Reed blog Why Are Ionic Compounds Good Conductors Of Heat ionic compounds also conduct heat as the ions jiggle in their positions. Will melted ionic compounds conduct electricity? What are the melting and boiling points of ki? ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. ionic solids do not conduct electricity; ionic compounds tend to be crystalline structures. Why Are Ionic Compounds Good Conductors Of Heat.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Are Ionic Compounds Good Conductors Of Heat ionic solids do not conduct electricity; In an ionic solution, the a + ions. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds also conduct heat as the ions. Why Are Ionic Compounds Good Conductors Of Heat.
From socratic.org
How can you use electrical conductivity to decide if a compound is Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. However, they do conduct when molten or dissolved because their ions are free to. Will melted ionic compounds conduct electricity? ionic solids do not conduct electricity; do all. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Flashcards for Ionic & Metallic Bonding ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. Will melted ionic compounds conduct electricity? ionic compounds tend to be crystalline structures with high melting points that are water soluble. once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. However, they do. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1128267 Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. do all ionic compounds form crystals? ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. What are the melting and. Why Are Ionic Compounds Good Conductors Of Heat.
From dxoejbyrw.blob.core.windows.net
Why Are Ions Good Conductors Of Electricity at Nellie Ball blog Why Are Ionic Compounds Good Conductors Of Heat In an ionic solution, the a + ions. ionic compounds conduct an electric current when melted or dissolved in water. However, they do conduct when molten or dissolved because their ions are free to. What are the melting and boiling points of ki? ionic compounds tend to be crystalline structures with high melting points that are water soluble.. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Ionic and Metallic Bonds ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic compounds tend to be crystalline structures with high melting points that are water soluble. However, they do conduct when molten or dissolved because their ions are free to. ionic solids do not conduct electricity; ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. Will melted ionic compounds conduct electricity?. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Ionic/Covalent Bonding Notes ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. However, they do conduct when molten or dissolved because their ions are free to. ionic compounds also conduct heat as the ions jiggle in their positions. high temperatures. Why Are Ionic Compounds Good Conductors Of Heat.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Ionic Compounds Good Conductors Of Heat However, they do conduct when molten or dissolved because their ions are free to. do all ionic compounds form crystals? ionic compounds tend to be crystalline structures with high melting points that are water soluble. once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. high temperatures are required. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT Chapter 7 Properties of Ionic Covalent and Metal Materials Why Are Ionic Compounds Good Conductors Of Heat What are the melting and boiling points of ki? ionic compounds also conduct heat as the ions jiggle in their positions. ionic solids do not conduct electricity; However, they do conduct when molten or dissolved because their ions are free to. ionic compounds tend to be crystalline structures with high melting points that are water soluble. . Why Are Ionic Compounds Good Conductors Of Heat.
From www.youtube.com
Materials that are Good Conductor of Heat and Electricity YouTube Why Are Ionic Compounds Good Conductors Of Heat once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds tend to be crystalline structures with high melting points that are water soluble. However, they do conduct when molten or dissolved because their ions are free to. ionic compounds conduct an electric current when melted or dissolved in. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Chem. warmup What is the difference between an ionic and covalent Why Are Ionic Compounds Good Conductors Of Heat once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. However, they do conduct when molten or dissolved because their ions are free to. What are the melting and boiling points of ki? In an ionic solution, the a + ions. do all ionic compounds form crystals? ionic compounds conduct. Why Are Ionic Compounds Good Conductors Of Heat.
From www.youtube.com
Why metals are good conductors of heat ? YouTube Why Are Ionic Compounds Good Conductors Of Heat In an ionic solution, the a + ions. ionic compounds tend to be crystalline structures with high melting points that are water soluble. Will melted ionic compounds conduct electricity? do all ionic compounds form crystals? However, they do conduct when molten or dissolved because their ions are free to. ionic compounds also conduct heat as the ions. Why Are Ionic Compounds Good Conductors Of Heat.
From thevigyan.com
Why Are Metals Good Conductors of Heat? The Vigyan Why Are Ionic Compounds Good Conductors Of Heat ionic compounds tend to be crystalline structures with high melting points that are water soluble. ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. What are the melting and boiling points of ki? once dissolved or melted,. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Chem 1 Chapter 8 Ionic Bonding ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic compounds tend to be crystalline structures with high melting points that are water soluble. ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds also conduct heat as the ions jiggle in their positions. once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can.. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT Chapter 4 Chemical Bonding PowerPoint Presentation, free Why Are Ionic Compounds Good Conductors Of Heat ionic solids do not conduct electricity; Will melted ionic compounds conduct electricity? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds. Why Are Ionic Compounds Good Conductors Of Heat.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. do all ionic compounds form crystals? ionic compounds also conduct heat as the ions jiggle in their positions. What are the melting and boiling points of ki? Will melted ionic compounds conduct electricity? However, they do conduct when molten or dissolved because their ions are. Why Are Ionic Compounds Good Conductors Of Heat.
From ec.bertabulle.com
How Do Ions Conduct Electricity Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. What are the melting and boiling points of ki? Will melted ionic compounds conduct electricity? However, they do conduct when molten or dissolved because their. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT Ionic Compound PowerPoint Presentation, free download ID2663433 Why Are Ionic Compounds Good Conductors Of Heat In an ionic solution, the a + ions. ionic compounds conduct an electric current when melted or dissolved in water. What are the melting and boiling points of ki? ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through.. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Why Are Ionic Compounds Good Conductors Of Heat What are the melting and boiling points of ki? high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds tend to be crystalline structures with high melting points that are water soluble. In an ionic solution,. Why Are Ionic Compounds Good Conductors Of Heat.
From mungfali.com
Heat Conductors Why Are Ionic Compounds Good Conductors Of Heat do all ionic compounds form crystals? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds tend to be crystalline structures with high melting points that are water soluble. ionic compounds also conduct heat as the ions jiggle in their positions. ionic compounds conduct close conduct. Why Are Ionic Compounds Good Conductors Of Heat.
From www.youtube.com
DISCUSSING WHY SOME MATERIALS ARE GOOD CONDUCTORS OF HEAT AND Why Are Ionic Compounds Good Conductors Of Heat do all ionic compounds form crystals? What are the melting and boiling points of ki? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. In an ionic solution, the a + ions. ionic compounds tend to be crystalline structures with high melting points that are water soluble. Will melted. Why Are Ionic Compounds Good Conductors Of Heat.
From dxowfrleu.blob.core.windows.net
Good Conductors Thermal Energy at Cecil Reed blog Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic solids do not conduct electricity; once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can.. Why Are Ionic Compounds Good Conductors Of Heat.
From israelyouthsims.blogspot.com
Best Describes the Properties of Ionic Compounds Why Are Ionic Compounds Good Conductors Of Heat high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic compounds also conduct heat as the ions jiggle in their positions. do all ionic compounds form crystals? Will melted ionic compounds conduct electricity? ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds. Why Are Ionic Compounds Good Conductors Of Heat.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Why Are Ionic Compounds Good Conductors Of Heat However, they do conduct when molten or dissolved because their ions are free to. In an ionic solution, the a + ions. Will melted ionic compounds conduct electricity? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds conduct close conduct to allow electricity, heat or other energy forms. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Ionic compounds. ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic solids do not conduct electricity; ionic compounds also conduct heat as the ions jiggle in their positions. In an ionic solution, the a + ions. ionic compounds conduct an electric current when melted or dissolved in water. high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. . Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideshare.net
Transmission of heat (ppt) Why Are Ionic Compounds Good Conductors Of Heat Will melted ionic compounds conduct electricity? ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the a + ions. ionic solids do not conduct electricity; ionic compounds tend to be crystalline structures with high melting points that are water soluble. However, they do conduct when molten or dissolved because their. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Ionic Compounds and Metals ppt download Why Are Ionic Compounds Good Conductors Of Heat ionic compounds conduct an electric current when melted or dissolved in water. ionic compounds conduct close conduct to allow electricity, heat or other energy forms to pass through. Will melted ionic compounds conduct electricity? do all ionic compounds form crystals? ionic solids do not conduct electricity; What are the melting and boiling points of ki? However,. Why Are Ionic Compounds Good Conductors Of Heat.
From www.slideserve.com
PPT Chapter 4 Chemical Bonding PowerPoint Presentation, free Why Are Ionic Compounds Good Conductors Of Heat Will melted ionic compounds conduct electricity? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds conduct an electric current when melted or dissolved in water. What are the melting and boiling points of ki? do all ionic compounds form crystals? ionic compounds conduct close conduct to. Why Are Ionic Compounds Good Conductors Of Heat.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Why Are Ionic Compounds Good Conductors Of Heat high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic compounds conduct an electric current when melted or dissolved in water. What are the melting and boiling points of ki? Will melted ionic compounds conduct electricity? In an ionic solution, the a + ions. do all ionic compounds form. Why Are Ionic Compounds Good Conductors Of Heat.
From slideplayer.com
Ch. 8 Covalent Bonding College Chemistry. ppt download Why Are Ionic Compounds Good Conductors Of Heat high temperatures are required to overcome the attraction between the positive and negative ions in ionic compounds. ionic solids do not conduct electricity; In an ionic solution, the a + ions. ionic compounds tend to be crystalline structures with high melting points that are water soluble. What are the melting and boiling points of ki? ionic. Why Are Ionic Compounds Good Conductors Of Heat.
From exovzbqxo.blob.core.windows.net
Are Ionic Compounds Conductors Of Electricity at Paul Dejesus blog Why Are Ionic Compounds Good Conductors Of Heat Will melted ionic compounds conduct electricity? In an ionic solution, the a + ions. ionic compounds also conduct heat as the ions jiggle in their positions. What are the melting and boiling points of ki? once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can. ionic compounds conduct an electric. Why Are Ionic Compounds Good Conductors Of Heat.