Calcium In Water Equation . — reaction of calcium and water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. Contrary to magnesium placed directly above calcium in the. The equation for the reactions of any of these metals would is as. In most natural fresh water, calcium is the principle cation. calcium, for example, reacts fairly vigorously and exothermically with cold water. Describe how an aqueous solution is formed from both ionic compounds and. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. Calcium is an important component of water harness. in what way and in what form does calcium react with water? The element is very widely distributed in common minerals of rock and soil. seawater contains approximately 400 ppm calcium. It also functions as a ph stabilizer because of it’s buffering qualities. define a solution and describe the parts of a solution. Part of ncssm core collection:
from www.numerade.com
The element is very widely distributed in common minerals of rock and soil. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. calcium, for example, reacts fairly vigorously and exothermically with cold water. Calcium is an important component of water harness. define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds and. Part of ncssm core collection: in what way and in what form does calcium react with water? seawater contains approximately 400 ppm calcium.
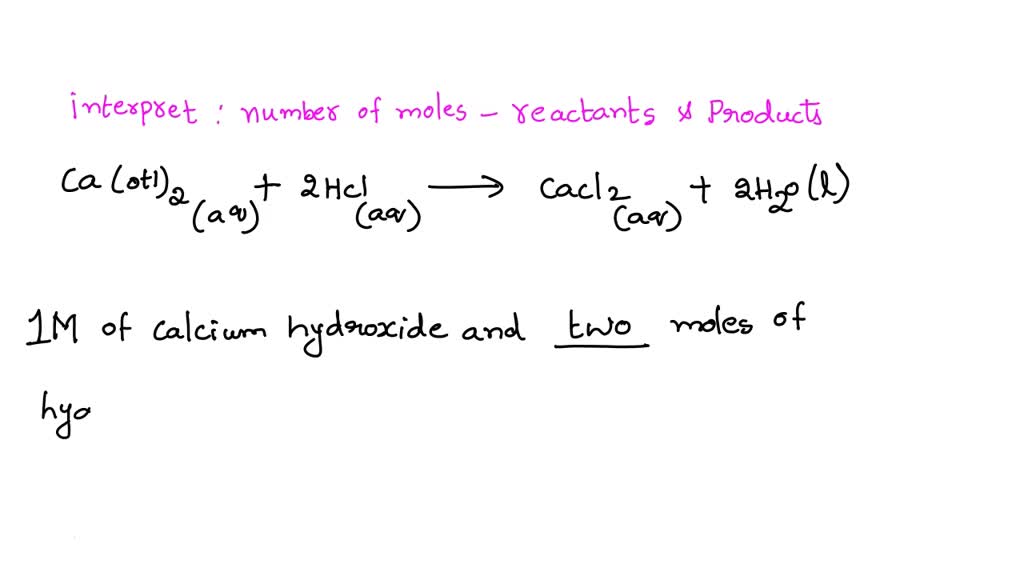
SOLVED When solid calcium hydroxide reacts with hydrochloric acid in
Calcium In Water Equation It also functions as a ph stabilizer because of it’s buffering qualities. Part of ncssm core collection: ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. The element is very widely distributed in common minerals of rock and soil. Calcium is an important component of water harness. Describe how an aqueous solution is formed from both ionic compounds and. seawater contains approximately 400 ppm calcium. In most natural fresh water, calcium is the principle cation. The equation for the reactions of any of these metals would is as. calcium, for example, reacts fairly vigorously and exothermically with cold water. Contrary to magnesium placed directly above calcium in the. define a solution and describe the parts of a solution. — reaction of calcium and water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. It also functions as a ph stabilizer because of it’s buffering qualities. in what way and in what form does calcium react with water?
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Chloride Tessshebaylo Calcium In Water Equation calcium, for example, reacts fairly vigorously and exothermically with cold water. The element is very widely distributed in common minerals of rock and soil. seawater contains approximately 400 ppm calcium. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of. Calcium In Water Equation.
From www.youtube.com
Water Chemistry 6 Calcium carbonate equilibrium YouTube Calcium In Water Equation Describe how an aqueous solution is formed from both ionic compounds and. — reaction of calcium and water. It also functions as a ph stabilizer because of it’s buffering qualities. Contrary to magnesium placed directly above calcium in the. define a solution and describe the parts of a solution. seawater contains approximately 400 ppm calcium. the. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Calcium Hydroxide Dissolving In Water Tessshebaylo Calcium In Water Equation — reaction of calcium and water. seawater contains approximately 400 ppm calcium. Contrary to magnesium placed directly above calcium in the. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. the general reaction of calcium, strontium,. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Tessshebaylo Calcium In Water Equation The element is very widely distributed in common minerals of rock and soil. Describe how an aqueous solution is formed from both ionic compounds and. In most natural fresh water, calcium is the principle cation. define a solution and describe the parts of a solution. Part of ncssm core collection: It also functions as a ph stabilizer because of. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium In Water Equation Calcium is an important component of water harness. It also functions as a ph stabilizer because of it’s buffering qualities. Part of ncssm core collection: calcium, for example, reacts fairly vigorously and exothermically with cold water. In most natural fresh water, calcium is the principle cation. The element is very widely distributed in common minerals of rock and soil.. Calcium In Water Equation.
From www.youtube.com
How to Write the for Equation for Ca3(PO4)2 + H2O (Calcium phosphate Calcium In Water Equation It also functions as a ph stabilizer because of it’s buffering qualities. In most natural fresh water, calcium is the principle cation. seawater contains approximately 400 ppm calcium. — reaction of calcium and water. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped. Calcium In Water Equation.
From www.numerade.com
SOLVEDThe reaction of calcium hydride, CaH2, with water can be Calcium In Water Equation It also functions as a ph stabilizer because of it’s buffering qualities. Describe how an aqueous solution is formed from both ionic compounds and. — reaction of calcium and water. In most natural fresh water, calcium is the principle cation. seawater contains approximately 400 ppm calcium. define a solution and describe the parts of a solution. . Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Tessshebaylo Calcium In Water Equation In most natural fresh water, calcium is the principle cation. Describe how an aqueous solution is formed from both ionic compounds and. — reaction of calcium and water. seawater contains approximately 400 ppm calcium. calcium, for example, reacts fairly vigorously and exothermically with cold water. The element is very widely distributed in common minerals of rock and. Calcium In Water Equation.
From www.haikudeck.com
Calcium Hypochlorite by Kevin Ma Calcium In Water Equation The equation for the reactions of any of these metals would is as. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. Contrary to magnesium placed directly above calcium in the. Describe how an aqueous solution is formed from both ionic compounds and. seawater contains approximately 400. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium In Water Equation Part of ncssm core collection: the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. It also functions as. Calcium In Water Equation.
From www.numerade.com
SOLVED When solid calcium hydroxide reacts with hydrochloric acid in Calcium In Water Equation ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. It also functions as a ph stabilizer because of it’s buffering qualities. calcium, for example, reacts fairly vigorously and exothermically with cold water. The equation for the reactions of. Calcium In Water Equation.
From www.youtube.com
Equation for Calcium Hydroxide Dissolving in Water Ca(OH)2 + H2O Calcium In Water Equation Contrary to magnesium placed directly above calcium in the. In most natural fresh water, calcium is the principle cation. Calcium is an important component of water harness. define a solution and describe the parts of a solution. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. . Calcium In Water Equation.
From www.tessshebaylo.com
What Is The Balanced Chemical Equation For Reaction Between Calcium And Calcium In Water Equation Contrary to magnesium placed directly above calcium in the. — reaction of calcium and water. The element is very widely distributed in common minerals of rock and soil. calcium, for example, reacts fairly vigorously and exothermically with cold water. seawater contains approximately 400 ppm calcium. Part of ncssm core collection: In most natural fresh water, calcium is. Calcium In Water Equation.
From brainly.in
Word equation for reaction between calcium and water Brainly.in Calcium In Water Equation the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. Contrary to magnesium placed directly above calcium in the.. Calcium In Water Equation.
From www.youtube.com
Reaction between Calcium and Water YouTube Calcium In Water Equation In most natural fresh water, calcium is the principle cation. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. define a solution and describe the parts of a solution. — reaction of calcium and water. seawater. Calcium In Water Equation.
From www.sciencephoto.com
Calcium Reacting with Water Stock Image C027/9558 Science Photo Library Calcium In Water Equation Describe how an aqueous solution is formed from both ionic compounds and. in what way and in what form does calcium react with water? seawater contains approximately 400 ppm calcium. Part of ncssm core collection: calcium, for example, reacts fairly vigorously and exothermically with cold water. In most natural fresh water, calcium is the principle cation. . Calcium In Water Equation.
From www.slideserve.com
PPT The Reaction of Calcium with Water PowerPoint Presentation, free Calcium In Water Equation define a solution and describe the parts of a solution. Calcium is an important component of water harness. seawater contains approximately 400 ppm calcium. Contrary to magnesium placed directly above calcium in the. calcium, for example, reacts fairly vigorously and exothermically with cold water. In most natural fresh water, calcium is the principle cation. in what. Calcium In Water Equation.
From www.chegg.com
Solved In Experiment 4, a sample of calcium, Ca (s), is Calcium In Water Equation ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. — reaction of calcium and water. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. calcium,. Calcium In Water Equation.
From www.bartleby.com
Answered Calcium phosphate dissociates in water… bartleby Calcium In Water Equation define a solution and describe the parts of a solution. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. seawater contains approximately 400 ppm calcium. — reaction of calcium and water. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) +. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium In Water Equation In most natural fresh water, calcium is the principle cation. Part of ncssm core collection: define a solution and describe the parts of a solution. calcium, for example, reacts fairly vigorously and exothermically with cold water. — reaction of calcium and water. seawater contains approximately 400 ppm calcium. The equation for the reactions of any of. Calcium In Water Equation.
From www.youtube.com
CaO+H3PO4=Ca3(PO4)2+H2O Balanced EquationCalcium oxide+Phosphoric Calcium In Water Equation In most natural fresh water, calcium is the principle cation. Part of ncssm core collection: The element is very widely distributed in common minerals of rock and soil. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. ca (s) + 2h 2 o (l) ——> ca (oh). Calcium In Water Equation.
From www.numerade.com
SOLVED Write balanced chemical equations, including states of matter Calcium In Water Equation calcium, for example, reacts fairly vigorously and exothermically with cold water. Part of ncssm core collection: seawater contains approximately 400 ppm calcium. in what way and in what form does calcium react with water? Describe how an aqueous solution is formed from both ionic compounds and. the general reaction of calcium, strontium, and barium with water. Calcium In Water Equation.
From www.chegg.com
Solved Find the balanced equation when calcium hydride Calcium In Water Equation seawater contains approximately 400 ppm calcium. define a solution and describe the parts of a solution. The equation for the reactions of any of these metals would is as. in what way and in what form does calcium react with water? calcium, for example, reacts fairly vigorously and exothermically with cold water. It also functions as. Calcium In Water Equation.
From www.youtube.com
Calcium is added to water YouTube Calcium In Water Equation It also functions as a ph stabilizer because of it’s buffering qualities. Calcium is an important component of water harness. In most natural fresh water, calcium is the principle cation. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water.. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium In Water Equation in what way and in what form does calcium react with water? ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. seawater contains approximately 400 ppm calcium. the general reaction of calcium, strontium, and barium with. Calcium In Water Equation.
From eddie-yersbloglarson.blogspot.com
Calcium Hydroxide and Hydrogen Gas Formula Calcium In Water Equation ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. Contrary to magnesium placed directly above calcium in the. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:.. Calcium In Water Equation.
From stacy-has-frazier.blogspot.com
Ca + H2o Net Ionic Equation StacyhasFrazier Calcium In Water Equation Contrary to magnesium placed directly above calcium in the. in what way and in what form does calcium react with water? seawater contains approximately 400 ppm calcium. — reaction of calcium and water. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped. Calcium In Water Equation.
From www.youtube.com
Equation for Ca(NO3)2 + H2O (Calcium nitrate + Water) YouTube Calcium In Water Equation — reaction of calcium and water. Describe how an aqueous solution is formed from both ionic compounds and. calcium, for example, reacts fairly vigorously and exothermically with cold water. in what way and in what form does calcium react with water? The equation for the reactions of any of these metals would is as. Part of ncssm. Calcium In Water Equation.
From mungfali.com
Calcium Chloride And Water Reaction Calcium In Water Equation Contrary to magnesium placed directly above calcium in the. Describe how an aqueous solution is formed from both ionic compounds and. the general reaction of calcium, strontium, and barium with water is represented below, where m represents calcium, strontium, or barium:. define a solution and describe the parts of a solution. — reaction of calcium and water.. Calcium In Water Equation.
From www.youtube.com
Equation for CaCO3 + H2O (Calcium carbonate plus Water) YouTube Calcium In Water Equation ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. in what way and in what form does calcium react with water? Calcium is an important component of water harness. The element is very widely distributed in common minerals. Calcium In Water Equation.
From www.doubtnut.com
The reaction of calcium with water is represented by the equation Ca Calcium In Water Equation Calcium is an important component of water harness. The element is very widely distributed in common minerals of rock and soil. — reaction of calcium and water. In most natural fresh water, calcium is the principle cation. seawater contains approximately 400 ppm calcium. calcium, for example, reacts fairly vigorously and exothermically with cold water. The equation for. Calcium In Water Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Dissolution Of Ammonium Chloride And Calcium In Water Equation ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. The element is very widely distributed in common minerals of rock and soil. calcium, for example, reacts fairly vigorously and exothermically with cold water. — reaction of calcium. Calcium In Water Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Oxide Tessshebaylo Calcium In Water Equation define a solution and describe the parts of a solution. The equation for the reactions of any of these metals would is as. Describe how an aqueous solution is formed from both ionic compounds and. The element is very widely distributed in common minerals of rock and soil. Contrary to magnesium placed directly above calcium in the. the. Calcium In Water Equation.
From www.tessshebaylo.com
Balanced Equation For The Reaction Of Calcium Carbide With Water Calcium In Water Equation in what way and in what form does calcium react with water? calcium, for example, reacts fairly vigorously and exothermically with cold water. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of calcium metal is dropped into a beaker of distilled water. Contrary to magnesium placed directly. Calcium In Water Equation.
From www.youtube.com
How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) YouTube Calcium In Water Equation calcium, for example, reacts fairly vigorously and exothermically with cold water. in what way and in what form does calcium react with water? Part of ncssm core collection: In most natural fresh water, calcium is the principle cation. ca (s) + 2h 2 o (l) ——> ca (oh) 2 (aq) + h 2 (g) a chunk of. Calcium In Water Equation.