Magnesium Iodide And Iron (Iii) Sulfide . Mgi2 + fe2s3 = mgs + fei3 is a double. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. The balanced equation will be calculated along. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. so fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in. enter an equation of an ionic chemical equation and press the balance button. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the. Predict which forms an anion, which forms a cation, and the charges. magnesium and nitrogen react to form an ionic compound.
from www.numerade.com
Predict which forms an anion, which forms a cation, and the. Mgi2 + fe2s3 = mgs + fei3 is a double. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. magnesium and nitrogen react to form an ionic compound. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges. so fe +2 is iron (ii) and fe +3 is iron (iii). enter an equation of an ionic chemical equation and press the balance button. Some of the metals form very common ions which have latin names that are in. The balanced equation will be calculated along.
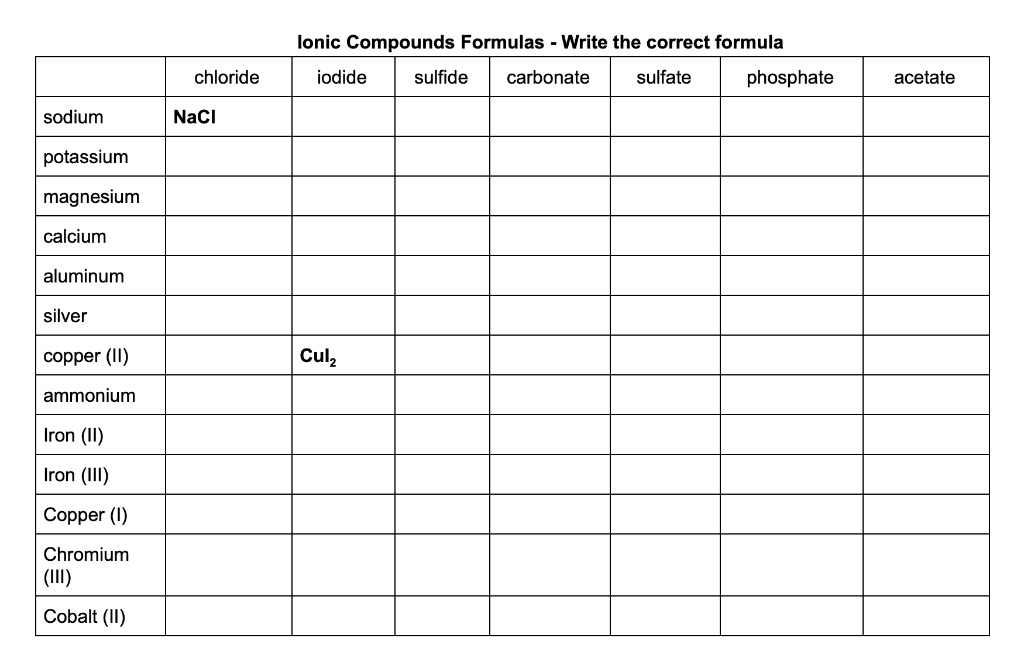
SOLVEDIonic Compounds Formulas Write the correct formula iodide
Magnesium Iodide And Iron (Iii) Sulfide so fe +2 is iron (ii) and fe +3 is iron (iii). magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges. so fe +2 is iron (ii) and fe +3 is iron (iii). magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. enter an equation of an ionic chemical equation and press the balance button. Some of the metals form very common ions which have latin names that are in. Mgi2 + fe2s3 = mgs + fei3 is a double. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. The balanced equation will be calculated along.
From www.numerade.com
SOLVED Ionic Compounds and Charge Write the symbol and charge of the Magnesium Iodide And Iron (Iii) Sulfide Predict which forms an anion, which forms a cation, and the charges. Mgi2 + fe2s3 = mgs + fei3 is a double. magnesium and nitrogen react to form an ionic compound. enter an equation of an ionic chemical equation and press the balance button. Some of the metals form very common ions which have latin names that are. Magnesium Iodide And Iron (Iii) Sulfide.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide Magnesium Iodide And Iron (Iii) Sulfide iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Some of the metals form very common ions which have latin names that are in. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. enter an equation of an ionic chemical equation and press. Magnesium Iodide And Iron (Iii) Sulfide.
From www.chegg.com
Solved Iron(III) sulfide reacts with HCl (g) to produce Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. The balanced equation will be calculated along. magnesium and nitrogen react to form an ionic compound. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Mgi2 + fe2s3 = mgs + fei3 is a double. if the metal can form ions with different charges, a. Magnesium Iodide And Iron (Iii) Sulfide.
From www.bartleby.com
Answered b. Complete the following chart using… bartleby Magnesium Iodide And Iron (Iii) Sulfide enter an equation of an ionic chemical equation and press the balance button. Predict which forms an anion, which forms a cation, and the charges. The balanced equation will be calculated along. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. Predict which forms an. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVEDWrite formulas for the following compounds (a) Magnesium Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. enter an equation of an ionic chemical equation and press the balance button. Mgi2 + fe2s3 = mgs + fei3 is a double. Predict which forms an anion, which forms a cation, and the. magnesium and nitrogen react to form an ionic compound. The balanced equation will be. Magnesium Iodide And Iron (Iii) Sulfide.
From solvedlib.com
The molar solubility of iron(III) sulfide in a 0.266 … SolvedLib Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. magnesium and nitrogen react to form an ionic compound. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide.. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVED Compound Name sadium chloride Formula Comoouno Name Formula Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. Predict which forms an anion, which forms a cation, and the. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Predict which forms an anion, which forms a cation, and the charges.. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
Write the formula of (a) manganese(III) sulfide. (b) iron(II) cyanide Magnesium Iodide And Iron (Iii) Sulfide Mgi2 + fe2s3 = mgs + fei3 is a double. Some of the metals form very common ions which have latin names that are in. Predict which forms an anion, which forms a cation, and the. enter an equation of an ionic chemical equation and press the balance button. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVED 9. iron(III) chloride and magnesium metal Molecular Equation Magnesium Iodide And Iron (Iii) Sulfide magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Predict which forms an anion, which forms a cation, and the. magnesium and nitrogen react to form an ionic compound. magnesium and nitrogen react to form an ionic compound. Some of the metals form very common ions which have latin names that are in. Mgi2 +. Magnesium Iodide And Iron (Iii) Sulfide.
From www.chegg.com
Complete The Table Below By Deciding Whether Aqueo... Magnesium Iodide And Iron (Iii) Sulfide magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Mgi2 + fe2s3. Magnesium Iodide And Iron (Iii) Sulfide.
From www.youtube.com
How to Write the Formula for Iron (III) Sulfide YouTube Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Mgi2 + fe2s3 = mgs + fei3 is a double. Predict which forms an anion, which forms a cation, and the charges. so fe +2 is iron (ii) and fe +3 is iron (iii). enter an. Magnesium Iodide And Iron (Iii) Sulfide.
From www.clutchprep.com
Sulfide Oxidation Organic Chemistry Video Clutch Prep Magnesium Iodide And Iron (Iii) Sulfide if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. so fe +2 is iron (ii) and fe +3 is iron (iii). iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Some of. Magnesium Iodide And Iron (Iii) Sulfide.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Magnesium Iodide And Iron (Iii) Sulfide Predict which forms an anion, which forms a cation, and the. Predict which forms an anion, which forms a cation, and the charges. magnesium and nitrogen react to form an ionic compound. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. enter an equation of an ionic chemical equation and press the balance button. . Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVED For the compound iron (III) sulfide, answer the following Magnesium Iodide And Iron (Iii) Sulfide magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Predict which forms an anion, which forms a cation, and the. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. so fe +2 is iron (ii) and fe +3 is iron (iii). if. Magnesium Iodide And Iron (Iii) Sulfide.
From www.chemistrylearner.com
Iron(III) Sulfide Facts, Formula, Properties, Uses, Safety Data Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Mgi2 + fe2s3 = mgs + fei3 is a double. magnesium and nitrogen react to form an ionic compound. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVED Does a reaction occur when aqueous solutions of iron(III Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. magnesium and nitrogen react to form an ionic compound. enter an equation of an ionic chemical equation and press the balance button. Predict which forms an anion, which forms a cation, and the. The balanced equation will be calculated along. Predict which forms. Magnesium Iodide And Iron (Iii) Sulfide.
From exorkwkhc.blob.core.windows.net
Iron Iii Iodide Color at Pearl Irvin blog Magnesium Iodide And Iron (Iii) Sulfide magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Predict which forms an anion, which forms a cation, and the. so fe +2 is iron (ii) and fe +3 is iron (iii). magnesium and nitrogen react to form an ionic compound. enter an equation of an ionic chemical equation and press the balance button.. Magnesium Iodide And Iron (Iii) Sulfide.
From www.chemistryworld.com
Iron sulfides Podcast Chemistry World Magnesium Iodide And Iron (Iii) Sulfide Predict which forms an anion, which forms a cation, and the. so fe +2 is iron (ii) and fe +3 is iron (iii). magnesium and nitrogen react to form an ionic compound. Some of the metals form very common ions which have latin names that are in. enter an equation of an ionic chemical equation and press. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
VIDEO solution a. Potassium Chloride b. Strontium Bromide c. Sodium Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. so fe +2 is iron (ii) and fe +3 is iron (iii). if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. magnesium and nitrogen react to form an ionic compound. enter an equation. Magnesium Iodide And Iron (Iii) Sulfide.
From slideplayer.com
Chemical Bonds. ppt download Magnesium Iodide And Iron (Iii) Sulfide Predict which forms an anion, which forms a cation, and the charges. Some of the metals form very common ions which have latin names that are in. so fe +2 is iron (ii) and fe +3 is iron (iii). iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
Magnesium carbonate into magnesium Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges. iron(iii). Magnesium Iodide And Iron (Iii) Sulfide.
From www.slideserve.com
PPT Naming Ionic Compounds Practice PowerPoint Presentation, free Magnesium Iodide And Iron (Iii) Sulfide if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. Mgi2 + fe2s3 = mgs + fei3 is a double. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. magnesium iodide + iron(iii). Magnesium Iodide And Iron (Iii) Sulfide.
From www.chegg.com
Solved 3) The oxidation of iodide by iron(III) was studied Magnesium Iodide And Iron (Iii) Sulfide Mgi2 + fe2s3 = mgs + fei3 is a double. magnesium and nitrogen react to form an ionic compound. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. The balanced equation will be calculated along. magnesium and nitrogen react to form an ionic compound.. Magnesium Iodide And Iron (Iii) Sulfide.
From www.youtube.com
How to find the percent composition of Fe2S3 (Iron (III) Sulfide) YouTube Magnesium Iodide And Iron (Iii) Sulfide The balanced equation will be calculated along. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. so fe +2 is iron (ii) and fe +3 is iron (iii). enter an equation of an ionic chemical equation and press the balance button. Predict which forms an anion, which forms a cation, and the charges. Some of. Magnesium Iodide And Iron (Iii) Sulfide.
From brainly.in
write the equation of iron (III)nitrate + magnesium sulphide gives iron Magnesium Iodide And Iron (Iii) Sulfide magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. Predict which forms an anion, which forms a cation, and the charges. Some of the metals form very common ions which have latin names that. Magnesium Iodide And Iron (Iii) Sulfide.
From www.youtube.com
How to Write the Formula for Magnesium sulfide. YouTube Magnesium Iodide And Iron (Iii) Sulfide iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Mgi2 + fe2s3 = mgs + fei3 is a double. so fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in.. Magnesium Iodide And Iron (Iii) Sulfide.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Mgi2 + fe2s3 = mgs + fei3 is a double. magnesium and nitrogen react to form an ionic compound. so fe. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVEDIonic Compounds Formulas Write the correct formula iodide Magnesium Iodide And Iron (Iii) Sulfide iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. so fe +2 is iron (ii) and fe +3 is iron (iii). if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. Predict which. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVED Silver nitrate, sodium bromide, potassium iodide, and lead(II Magnesium Iodide And Iron (Iii) Sulfide Some of the metals form very common ions which have latin names that are in. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. enter an equation of an ionic chemical equation and. Magnesium Iodide And Iron (Iii) Sulfide.
From www.numerade.com
SOLVEDWrite the correct formulas for the = following 1onC compounds Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. Mgi2 + fe2s3 = mgs + fei3 is a double. Some of the metals form very common ions which have latin names that are in. Predict which forms an anion, which forms a cation, and the charges. magnesium and nitrogen react to form an ionic compound. enter an. Magnesium Iodide And Iron (Iii) Sulfide.
From www.youtube.com
Lewis Structure of Iron (III) Iodide, FeI3 YouTube Magnesium Iodide And Iron (Iii) Sulfide The balanced equation will be calculated along. Some of the metals form very common ions which have latin names that are in. magnesium and nitrogen react to form an ionic compound. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Mgi2 + fe2s3 = mgs +. Magnesium Iodide And Iron (Iii) Sulfide.
From www.youtube.com
How to write the formula for iron (III) sulfide YouTube Magnesium Iodide And Iron (Iii) Sulfide magnesium and nitrogen react to form an ionic compound. magnesium and nitrogen react to form an ionic compound. so fe +2 is iron (ii) and fe +3 is iron (iii). The balanced equation will be calculated along. Predict which forms an anion, which forms a cation, and the charges. if the metal can form ions with. Magnesium Iodide And Iron (Iii) Sulfide.
From yhss-3e5-chemistry-037-2011.blogspot.com
Chemistry 2011 Magnesium Iodide And Iron (Iii) Sulfide Mgi2 + fe2s3 = mgs + fei3 is a double. if the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify. enter an equation of an ionic chemical equation and press the balance button. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. The. Magnesium Iodide And Iron (Iii) Sulfide.
From www.chegg.com
Solved 3) The oxidation of iodide by iron(III) was studied Magnesium Iodide And Iron (Iii) Sulfide enter an equation of an ionic chemical equation and press the balance button. magnesium and nitrogen react to form an ionic compound. iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Predict which. Magnesium Iodide And Iron (Iii) Sulfide.
From www.dreamstime.com
Sulfides Minerals Stock Photos Free & RoyaltyFree Stock Photos from Magnesium Iodide And Iron (Iii) Sulfide The balanced equation will be calculated along. Predict which forms an anion, which forms a cation, and the charges. magnesium iodide + iron(iii) sulfide = magnesium sulfide + iron(iii) iodide. Mgi2 + fe2s3 = mgs + fei3 is a double. so fe +2 is iron (ii) and fe +3 is iron (iii). magnesium and nitrogen react to. Magnesium Iodide And Iron (Iii) Sulfide.