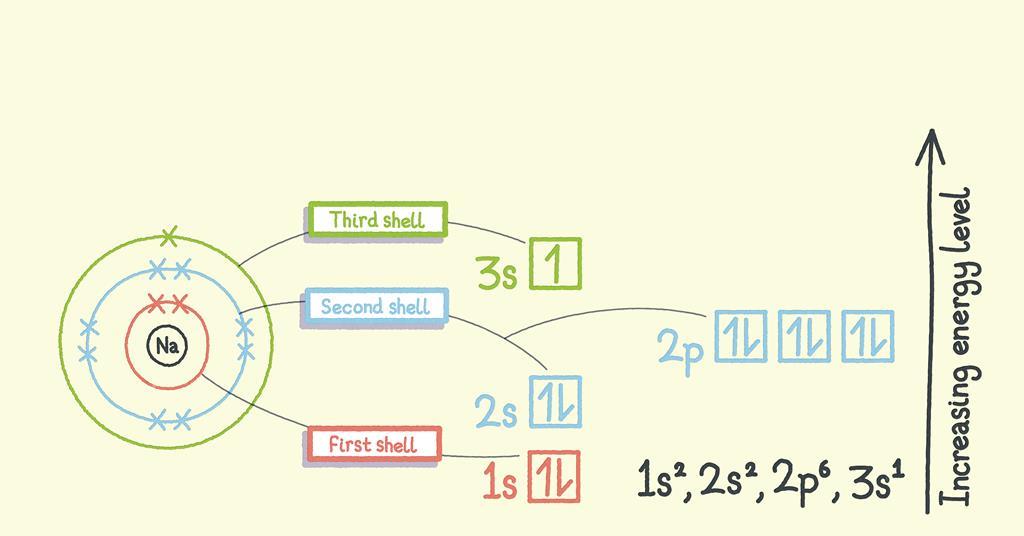
Structure Of Subshell . The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The 4s subshell is filled before the 3d subshell. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. (b) the three p orbitals have two lobes, shaped. 18.3 structure and general properties of the metalloids; This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. This is reflected in the structure of the periodic table.
from edu.rsc.org
After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (b) the three p orbitals have two lobes, shaped. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; The 4s subshell is filled before the 3d subshell. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. This is reflected in the structure of the periodic table.
How to teach electron configurations Poster RSC Education
Structure Of Subshell The 4s subshell is filled before the 3d subshell. 18.4 structure and general properties of the nonmetals; This is reflected in the structure of the periodic table. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The 4s subshell is filled before the 3d subshell. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (b) the three p orbitals have two lobes, shaped. (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap.
From www.globalsino.com
Electron Subshells Structure Of Subshell This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; 18.4 structure and general properties of the nonmetals; (b) the. Structure Of Subshell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. 18.3 structure and general properties of the metalloids; This is reflected in the structure of the periodic table. The arrangement of electrons in the orbitals of an atom is called the electron configuration. Structure Of Subshell.
From manuallistcantabank.z21.web.core.windows.net
Chemistry Orbital Diagram Structure Of Subshell The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. The 4s subshell is filled before the 3d subshell. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. (b) the three p. Structure Of Subshell.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Structure Of Subshell 18.3 structure and general properties of the metalloids; The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (a) the lone s orbital in an s subshell is spherical in distribution. This is reflected in the structure of the periodic table. (b) the three p orbitals have two lobes, shaped. The 4s. Structure Of Subshell.
From www.researchgate.net
He(II) UPS spectra of the QH (top panel) and LL (bottom panel Structure Of Subshell The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (b) the three p orbitals have two lobes, shaped. The 4s subshell is filled before the 3d subshell. (a) the lone s orbital in an s subshell is spherical in distribution. This video explains the concepts of shells, subshells, and orbitals in. Structure Of Subshell.
From www.e-magnetica.pl
[Encyclopedia Structure Of Subshell (b) the three p orbitals have two lobes, shaped. This is reflected in the structure of the periodic table. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The 4s subshell is filled before the 3d subshell. (a) the lone s orbital in an s subshell is spherical in distribution. This. Structure Of Subshell.
From edu.rsc.org
How to teach electron configurations Poster RSC Education Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. 18.3 structure and general properties of the metalloids; After the 4s subshell is filled, the 3d subshell is. Structure Of Subshell.
From lavelle.chem.ucla.edu
s,p,d,f orbital characteristics? CHEMISTRY COMMUNITY Structure Of Subshell 18.4 structure and general properties of the nonmetals; After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (a) the lone s orbital in an s subshell is spherical in distribution. This is reflected in the structure of the periodic table. This video explains the concepts of shells, subshells, and orbitals in atomic structure. Structure Of Subshell.
From www.savemyexams.com
Electron Subshells & Orbitals (1.1.7) CIE A Level Chemistry Revision Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The 4s subshell is filled before the 3d subshell. (b) the three p orbitals have two lobes, shaped. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. 18.4 structure and general properties of the nonmetals; 18.3 structure and general. Structure Of Subshell.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (a) the lone s orbital in an s subshell is spherical in distribution. (b) the three p orbitals have two lobes, shaped. 18.4 structure and general properties of. Structure Of Subshell.
From schematicellsshyers.z21.web.core.windows.net
How To Do Orbital Diagrams Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. This is reflected in the structure of the periodic table. 18.4 structure and general properties of the nonmetals; 18.3 structure and general properties of the metalloids; After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. This video explains the concepts. Structure Of Subshell.
From www.breakingatom.com
Subshells Definition Structure Of Subshell 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. This is reflected in the structure of the periodic table. The 4s subshell is filled before the 3d subshell. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. The arrangement of electrons. Structure Of Subshell.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.6 Electronic Structure翰林国际教育 Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. 18.4 structure and general properties of the nonmetals; (b) the three p orbitals have two lobes, shaped. The arrangement of electrons in the orbitals. Structure Of Subshell.
From psu.pb.unizin.org
2.1 Atoms, Isotopes, Ions, and Molecules The Building Blocks Structure Of Subshell 18.4 structure and general properties of the nonmetals; 18.3 structure and general properties of the metalloids; After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (a) the lone s orbital in an s subshell is spherical in distribution. (b) the three p orbitals have two lobes, shaped. The 4s subshell is filled before. Structure Of Subshell.
From schematicfixheartly.z22.web.core.windows.net
Orbital Diagram Electron Configuration Structure Of Subshell The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (b) the three p orbitals have two lobes, shaped. This is reflected in the structure of the periodic table. 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. After the. Structure Of Subshell.
From gioxaqmhp.blob.core.windows.net
What Is Electron Configuration Periodic Table at Debbie Chupp blog Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (b) the three p orbitals have two lobes, shaped. After the 4s subshell is filled, the 3d subshell is filled with. Structure Of Subshell.
From chem.libretexts.org
2.2 Electronic Structure of Atoms Chemistry LibreTexts Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. (a) the lone s orbital in an s subshell is spherical in distribution. The 4s subshell is filled before the 3d subshell. 18.3 structure and general properties of the metalloids; (b) the three p orbitals have two lobes, shaped. 18.4 structure and general properties of the. Structure Of Subshell.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry Structure Of Subshell The 4s subshell is filled before the 3d subshell. (b) the three p orbitals have two lobes, shaped. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. After the 4s subshell is filled, the 3d subshell is. Structure Of Subshell.
From phys.libretexts.org
8.5 The Exclusion Principle and the Periodic Table Physics LibreTexts Structure Of Subshell 18.3 structure and general properties of the metalloids; (a) the lone s orbital in an s subshell is spherical in distribution. This is reflected in the structure of the periodic table. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (b) the three p orbitals have two lobes, shaped. Learn about atomic structure. Structure Of Subshell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Structure Of Subshell 18.3 structure and general properties of the metalloids; This is reflected in the structure of the periodic table. 18.4 structure and general properties of the nonmetals; (b) the three p orbitals have two lobes, shaped. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The 4s subshell is filled before the. Structure Of Subshell.
From socratic.org
L=4 subshell?? Socratic Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. (a) the lone s orbital in an s subshell is spherical in distribution. 18.4 structure. Structure Of Subshell.
From www.youtube.com
[AS Chemistry] Atomic Structure Shells, Subshells, and Orbitals YouTube Structure Of Subshell The 4s subshell is filled before the 3d subshell. (a) the lone s orbital in an s subshell is spherical in distribution. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. 18.4 structure and general. Structure Of Subshell.
From www.vrogue.co
How Many Orbitals Are There In D Subshell Chemistry S vrogue.co Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. (a) the lone s orbital in an s subshell is spherical in distribution. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. The 4s subshell is filled before the 3d subshell. 18.3 structure and general properties of the metalloids;. Structure Of Subshell.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Structure Of Subshell The 4s subshell is filled before the 3d subshell. 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. 18.3 structure and general properties of the metalloids; After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. Learn about atomic structure and electron. Structure Of Subshell.
From circuitenginephyle101.z19.web.core.windows.net
How To Read Orbital Diagrams Structure Of Subshell 18.4 structure and general properties of the nonmetals; The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. (a) the lone s orbital in an s subshell is spherical in distribution. The 4s subshell is filled before the 3d subshell. Learn about atomic structure and electron configuration through khan academy's instructional video. Structure Of Subshell.
From www.savemyexams.com
Photoelectron Spectroscopy College Board AP® Chemistry Study Guides 2022 Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The 4s subshell is filled before the 3d subshell. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (a) the lone s orbital in. Structure Of Subshell.
From www.peoi.org
Chapter 8 Section C Organization of Electrons in Atoms Structure Of Subshell This is reflected in the structure of the periodic table. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. The 4s subshell is filled before the 3d subshell. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. (b) the three p orbitals have two lobes, shaped. This video. Structure Of Subshell.
From mungfali.com
Shell Vs Subshell Structure Of Subshell The 4s subshell is filled before the 3d subshell. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. This is reflected in the structure of the periodic table. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. 18.3 structure and general properties of the metalloids; This. Structure Of Subshell.
From mapma.in
How Electronic Configuration is Done A Comprehensive Guide Electro Glow Structure Of Subshell 18.3 structure and general properties of the metalloids; 18.4 structure and general properties of the nonmetals; The 4s subshell is filled before the 3d subshell. (b) the three p orbitals have two lobes, shaped. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. (a) the lone s orbital in an s subshell is spherical. Structure Of Subshell.
From www.britannica.com
Electron shell Definition & Facts Britannica Structure Of Subshell This is reflected in the structure of the periodic table. 18.4 structure and general properties of the nonmetals; (a) the lone s orbital in an s subshell is spherical in distribution. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. This video explains the concepts of shells, subshells, and orbitals in atomic structure. Structure Of Subshell.
From socratic.org
What is the difference between a shell and a subshell? Socratic Structure Of Subshell After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. (a) the lone s orbital in an s subshell is spherical in distribution. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The arrangement. Structure Of Subshell.
From giotggcaz.blob.core.windows.net
Which Electron Shell Has The Highest Energy Level at Virginia Washburn blog Structure Of Subshell (a) the lone s orbital in an s subshell is spherical in distribution. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The 4s subshell is filled before the 3d subshell. 18.3 structure and general properties of the metalloids; 18.4. Structure Of Subshell.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure Structure Of Subshell This is reflected in the structure of the periodic table. 18.4 structure and general properties of the nonmetals; This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. (a) the lone s orbital in an s subshell is spherical in distribution. After the 4s subshell is filled, the 3d subshell is filled with up to. Structure Of Subshell.
From www.pinterest.com
This is a periodic table in which the elements are ordered by the Structure Of Subshell The 4s subshell is filled before the 3d subshell. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. This video explains the concepts of shells, subshells, and orbitals in atomic structure for ap. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (a) the. Structure Of Subshell.
From mungfali.com
Orbital Subshell Chart Structure Of Subshell Learn about atomic structure and electron configuration through khan academy's instructional video on orbitals. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. After the 4s subshell is filled, the 3d subshell is filled with up to 10 electrons. (b) the three p orbitals have two lobes, shaped. This is reflected. Structure Of Subshell.