Crystal Lattice Meaning . The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. Most solids have periodic arrays of atoms which form what we call a crystal lattice. For example, table salt is composed of sodium and. Determining atomic radius from density, molar mass and crystal structure. Amorphous solids and glasses are exceptions. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal.
from eduinput.com
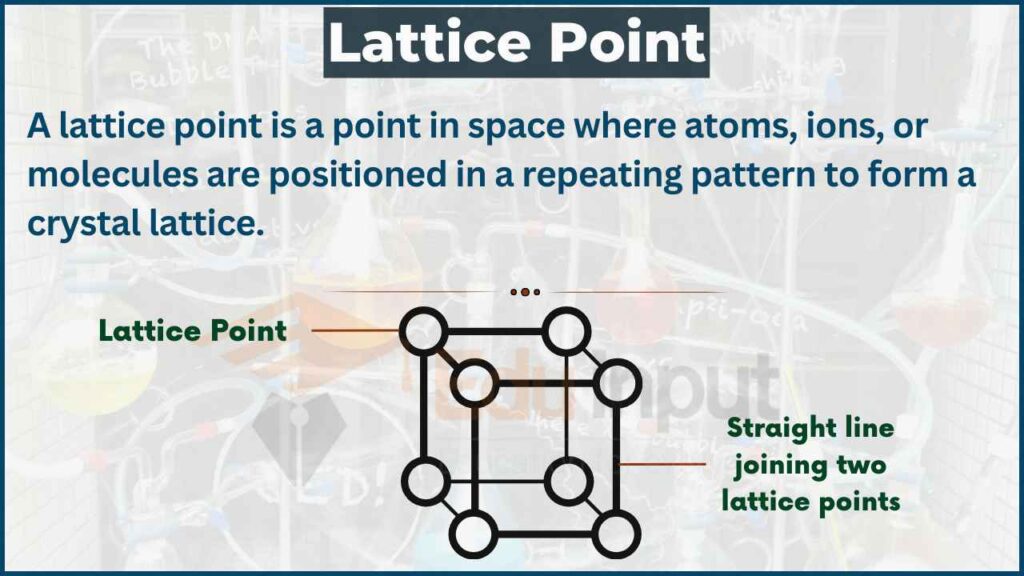
A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Determining atomic radius from density, molar mass and crystal structure. Most solids have periodic arrays of atoms which form what we call a crystal lattice. For example, table salt is composed of sodium and. Amorphous solids and glasses are exceptions. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid.
Crystal lattice Definition, Types, examples, lattice point
Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. For example, table salt is composed of sodium and. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Most solids have periodic arrays of atoms which form what we call a crystal lattice. Determining atomic radius from density, molar mass and crystal structure. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. Amorphous solids and glasses are exceptions. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos.
From www.youtube.com
Lattice, Basis, Crystal System Crystal Structure Solid State Crystal Lattice Meaning Amorphous solids and glasses are exceptions. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. For example, table salt is composed of sodium and. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is a highly ordered repeating pattern of atoms or. Crystal Lattice Meaning.
From www.slideserve.com
PPT Unit1 Crystal Structure & Bravais lattice . PowerPoint Crystal Lattice Meaning The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. A crystal structure is a unique arrangement of atoms, ions or molecules in a. Crystal Lattice Meaning.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystal Lattice Meaning Determining atomic radius from density, molar mass and crystal structure. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. For example, table salt is composed of sodium and. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Most solids have periodic arrays of atoms. Crystal Lattice Meaning.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystal Lattice Meaning For example, table salt is composed of sodium and. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Amorphous solids and glasses are exceptions. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal lattice is an ordered array. Crystal Lattice Meaning.
From home.iitk.ac.in
Basic Crystal Concepts Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Amorphous solids and glasses are exceptions. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. For example, table salt is composed of sodium and.. Crystal Lattice Meaning.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystal Lattice Meaning The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. A crystal lattice is. Crystal Lattice Meaning.
From school.careers360.com
crystal lattices and unit cells Overview, Structure, Properties & Uses Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. Most solids have periodic arrays of atoms which form what we call a crystal lattice. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. The strategy is. Crystal Lattice Meaning.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Determining atomic radius from density, molar mass and crystal structure. Most solids have periodic arrays of atoms which form what we call a crystal lattice. The strategy is to use the following to calculate the volume of the unit cell, and then. Crystal Lattice Meaning.
From www.graylark.com
Crystal Lattices Crystal Lattice Meaning Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. For example, table salt is composed of sodium and. Amorphous solids and glasses are exceptions. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal.. Crystal Lattice Meaning.
From www.youtube.com
CRYSTAL LATTICE, LATTICE POINT, UNIT CELL SOLID STATE PART 3 Crystal Lattice Meaning The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. For example, table salt is composed of sodium and. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A crystal lattice is a highly ordered repeating pattern of atoms. Crystal Lattice Meaning.
From www.britannica.com
Crystallography Definition & Facts Britannica Crystal Lattice Meaning A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Amorphous solids and glasses are exceptions. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Most solids have periodic arrays of atoms. Crystal Lattice Meaning.
From www.slideserve.com
PPT Unit VII Crystal structure PowerPoint Presentation ID1426222 Crystal Lattice Meaning A crystal lattice is a highly ordered repeating pattern of atoms or molecules. For example, table salt is composed of sodium and. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side.. Crystal Lattice Meaning.
From mungfali.com
Crystal Lattice Structure Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. For example, table salt is composed of sodium and. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. A crystal lattice is. Crystal Lattice Meaning.
From studylib.net
The Crystal Lattice Crystal Lattice Meaning Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Amorphous solids and glasses are exceptions. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. For example, table salt is composed of sodium and. The strategy is to use the following to calculate the volume. Crystal Lattice Meaning.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn about. Crystal Lattice Meaning.
From www.slideserve.com
PPT Unit VII Crystal structure PowerPoint Presentation, free download Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Determining atomic radius from density, molar mass. Crystal Lattice Meaning.
From bernieralice.hyperphp.com
Types Of Crystal Lattice Structure bernieralice Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Determining atomic radius from. Crystal Lattice Meaning.
From www.pinterest.com
The Crystal Lattice Structure of Ionic Compounds infographic diagram Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A crystal. Crystal Lattice Meaning.
From study.com
Crystal Lattice Definition & Structure Video & Lesson Transcript Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Most solids have periodic arrays of atoms which form what we call a crystal lattice. Determining atomic radius from density, molar mass and crystal structure. Amorphous. Crystal Lattice Meaning.
From nanohub.org
Resources ECE 606 Lecture 2 Geometry of Periodic Crystal Lattice Meaning For example, table salt is composed of sodium and. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Learn about ionic, covalent,. Crystal Lattice Meaning.
From www.tec-science.com
Important types of lattice structures tecscience Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn the definition, characteristics and types of crystal lattices. Crystal Lattice Meaning.
From www.graylark.com
Crystal Lattices Crystal Lattice Meaning A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Determining atomic radius from density, molar mass and crystal structure. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. A crystal lattice. Crystal Lattice Meaning.
From www.pinterest.com
crystal Crystals, Lattice, Geology rocks Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. For example, table salt is composed of sodium and. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Most solids have periodic arrays of atoms which form what we call a crystal lattice. Determining atomic radius from density, molar mass. Crystal Lattice Meaning.
From www.slideserve.com
PPT Unit1 Crystal Structure & Bravais lattice . PowerPoint Crystal Lattice Meaning Most solids have periodic arrays of atoms which form what we call a crystal lattice. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Amorphous solids and glasses are exceptions. A crystal lattice. Crystal Lattice Meaning.
From psiberg.com
Crystal Structures Types with Explanation PSIBERG Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. For example, table salt is composed of sodium and.. Crystal Lattice Meaning.
From courses.lumenlearning.com
Lattice Structures in Crystalline Solids Chemistry for Majors Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Amorphous solids and glasses are exceptions. For example, table salt is composed of sodium and. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. The strategy is to use the following to calculate the volume of the unit. Crystal Lattice Meaning.
From helecu.com
The 7 Crystal Systems (with Examples and Images) Materials Science Crystal Lattice Meaning Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Most solids have periodic arrays of atoms which form what we call a crystal lattice. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. A crystal lattice is an ordered array. Crystal Lattice Meaning.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. For example, table salt is composed of sodium and. Most solids have periodic arrays of atoms which form what we call a crystal lattice. A crystal structure is a unique arrangement. Crystal Lattice Meaning.
From eduinput.com
Difference between crystal lattice and lattice point Crystal Lattice Meaning Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. For example, table salt is composed of sodium and. A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Most solids have periodic arrays of atoms which form what we call a crystal lattice. A crystal structure is a unique arrangement. Crystal Lattice Meaning.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. For example, table salt is composed of sodium and. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. The strategy is to use the following to calculate the volume of the unit cell, and then the length of. Crystal Lattice Meaning.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystal Lattice Meaning For example, table salt is composed of sodium and. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn the definition, characteristics and types of crystal lattices and unit cells with examples and videos. Determining atomic radius from density, molar mass and crystal structure. Most solids have. Crystal Lattice Meaning.
From www.pinterest.com
Crystal Lattice Easy Science Crystal lattice, Dimensional patterns Crystal Lattice Meaning Amorphous solids and glasses are exceptions. For example, table salt is composed of sodium and. A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. Most solids have periodic arrays of atoms which form what we call a crystal lattice. The strategy is to use the following to calculate the volume of. Crystal Lattice Meaning.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Crystal Lattice Meaning A crystal lattice is an ordered array of points describing the arrangement of particles that form a crystal. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. The strategy is to use the following to calculate the volume of the unit cell, and then the. Crystal Lattice Meaning.
From www.slideserve.com
PPT Chapter 6 Chemical Bonds PowerPoint Presentation, free download Crystal Lattice Meaning Amorphous solids and glasses are exceptions. A crystal lattice is a highly ordered repeating pattern of atoms or molecules. Determining atomic radius from density, molar mass and crystal structure. Most solids have periodic arrays of atoms which form what we call a crystal lattice. The strategy is to use the following to calculate the volume of the unit cell, and. Crystal Lattice Meaning.
From www.britannica.com
Hexagonal closepacked structure crystallography Britannica Crystal Lattice Meaning Determining atomic radius from density, molar mass and crystal structure. Most solids have periodic arrays of atoms which form what we call a crystal lattice. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Learn about ionic, covalent, and bravais lattices, lattice constants, and lattice. For example,. Crystal Lattice Meaning.