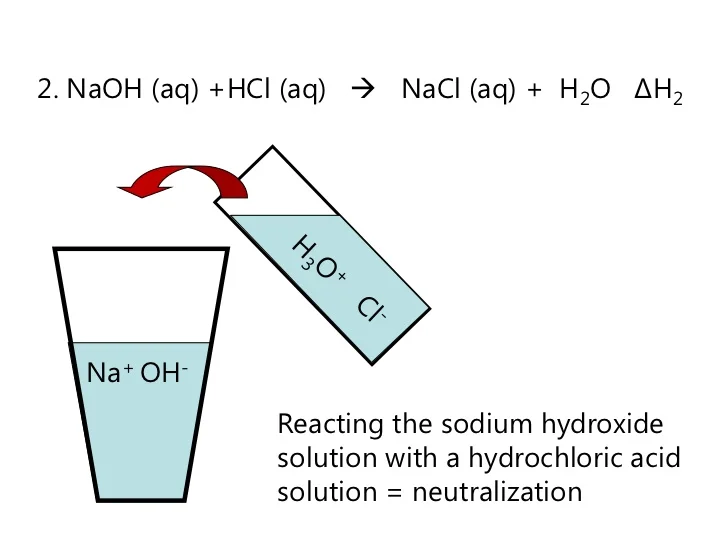
Examples Of Sodium Hydroxide And Hydrochloric Acid . To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Includes kit list and safety instructions. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the.
from www.slideshare.net
The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.
5 3 and 5 4
Examples Of Sodium Hydroxide And Hydrochloric Acid The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Includes kit list and safety instructions. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6054682 Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Includes kit list and. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From indianewsmack.blogspot.com
Hydrochloric Acid and Sodium Hydroxide Balanced Equation With States Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When equal amounts of a. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Examples Of Sodium Hydroxide And Hydrochloric Acid Includes kit list and safety instructions. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. For example, zinc metal reacts with hydrochloric acid, producing zinc. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.sciencephoto.com
Sodium Hydroxide Reacts with Hydrochloric Acid Stock Image C036 Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Includes kit list and safety instructions. Use this class practical to explore titration, producing the. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From stock.adobe.com
Vetor de illustration of chemistry, Acids, salt and bases, Hydrogen Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The equation for any strong acid being neutralized by a strong alkali is essentially just. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When equal amounts of a strong acid such as hydrochloric acid are mixed. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From studylib.net
Hydrochloric acid and sodium hydroxide are the most common Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube Examples Of Sodium Hydroxide And Hydrochloric Acid Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. To understand the full extent of the. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.alamy.com
Table salt hydrochloric acid and sodium hydroxide Stock Photo Alamy Examples Of Sodium Hydroxide And Hydrochloric Acid Includes kit list and safety instructions. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
How to Balance NaOH + HCl = NaCl + H2O (Sodium Hydroxide Plus Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. The equation for any strong acid being neutralized. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From stock.adobe.com
Acidbase reaction. chemical reaction neutralization. HCl hydrochloric Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. For example, zinc metal reacts. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.mediabakery.com
Mediabakery Photo by Science Photo Library Illustration of an acid Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. When hydrochloric acid. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From basdemax.netlify.app
28++ Hydrochloric Acid And Sodium Hydroxide Balanced Equation Basdemax Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When hydrochloric acid (hcl) and sodium hydroxide. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.slideshare.net
5 3 and 5 4 Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Includes kit list and safety instructions. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From mavink.com
Hydrochloric Acid React With Sodium Hydroxide Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Sodium hydroxide reaction with hydrochloric acid Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From indianewsmack.blogspot.com
Hydrochloric Acid and Sodium Hydroxide Balanced Equation With States Examples Of Sodium Hydroxide And Hydrochloric Acid Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From insende.netlify.app
33++ Hydrochloric Acid And Sodium Hydroxide Reaction Insende Examples Of Sodium Hydroxide And Hydrochloric Acid Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. For example, zinc metal reacts with hydrochloric. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Examples Of Sodium Hydroxide And Hydrochloric Acid Includes kit list and safety instructions. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When equal amounts of a strong acid such as hydrochloric acid are mixed with a. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Neutralisation Reaction of Sodium hydroxide and Hydrochloric acid YouTube Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From slidetodoc.com
Starter complete the equations Hydrochloric acid sodium hydroxide Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Includes kit list and safety instructions. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Use this class practical to explore titration, producing the. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From stock.adobe.com
Vector illustration of electrolytic dissociation. Molecules break up Examples Of Sodium Hydroxide And Hydrochloric Acid Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. When equal amounts of a strong acid. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.dreamstime.com
Neutralization Reaction of Hydrochloric Acid and Sodium Hydroxid Stock Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Includes. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Examples Of Sodium Hydroxide And Hydrochloric Acid The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to explore. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Sodium hydroxide and hydrochloric acid YouTube Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. The equation for any strong acid being neutralized by a strong alkali. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From brainly.com
Below is the word equation for the reaction between sodium hydroxide Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. For example, zinc metal reacts with. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.youtube.com
Making Sodium Hydroxide and Hydrochloric Acid from salt water. YouTube Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. Includes kit list and safety instructions. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From fphoto.photoshelter.com
neutralization hydrochloric acid sodium hydroxide chemistry Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From slidetodoc.com
Salts Soluble Insoluble Salts All sodium potassium and Examples Of Sodium Hydroxide And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. The equation for any strong acid being neutralized by. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From thisnutrition.com
Hydrochloric Acid And Sodium Hydroxide This Nutrition Examples Of Sodium Hydroxide And Hydrochloric Acid When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. For. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From askfilo.com
The reaction of hydrochloric acid with sodium hydroxide is a example of.. Examples Of Sodium Hydroxide And Hydrochloric Acid The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From www.shalom-education.com
Acids and Alkalis GCSE Chemistry Revision Examples Of Sodium Hydroxide And Hydrochloric Acid Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. To understand the full extent of the tragedy, let’s get better. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. The equation for any strong acid being neutralized by a strong alkali is essentially just a reaction between hydrogen ions and. Includes kit list and safety instructions. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed. Examples Of Sodium Hydroxide And Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Examples Of Sodium Hydroxide And Hydrochloric Acid To understand the full extent of the tragedy, let’s get better acquainted with them and examine the nature of sodium hydroxide and hydrochloric. When hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form sodium. Includes kit list and safety instructions. The equation for any strong acid being neutralized by a strong alkali. Examples Of Sodium Hydroxide And Hydrochloric Acid.