Process Buffer Range . The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. Has the largest buffer capacity for its concentration). A weak acid and its conjugate base, or b. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Buffers function through a process of chemical equilibrium. Once this range has been exceeded. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. The buffer range is the range of ph values over which a buffer is most effective (i.e. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve.
from www.alfalaval.in
A weak acid and its conjugate base, or b. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The buffer range is the range of ph values over which a buffer is most effective (i.e. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. Buffers function through a process of chemical equilibrium. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Once this range has been exceeded. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it.
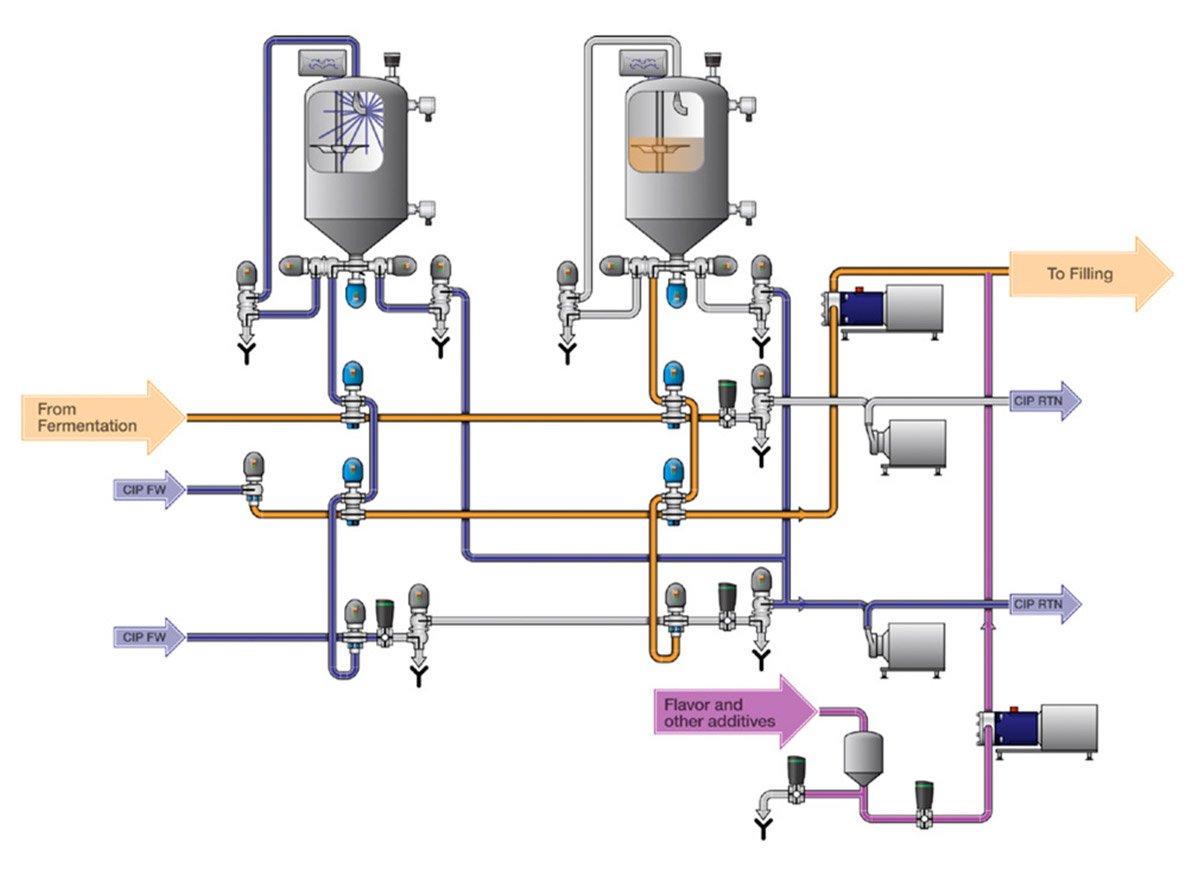
Alfa Laval Buffer storage
Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Once this range has been exceeded. A weak acid and its conjugate base, or b. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Has the largest buffer capacity for its concentration). The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers function through a process of chemical equilibrium.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Process Buffer Range Once this range has been exceeded. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production. Process Buffer Range.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Process Buffer Range The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. Once this range has been exceeded. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A weak acid. Process Buffer Range.
From www.youtube.com
Role of buffers in biological system YouTube Process Buffer Range A weak acid and its conjugate base, or b. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Once this range has been exceeded. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in. Process Buffer Range.
From saylordotorg.github.io
Buffers Process Buffer Range Once this range has been exceeded. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Has the largest buffer capacity for its concentration). A weak acid and its. Process Buffer Range.
From www.dojindo.com
Good's Buffer ACES DOJINDO Process Buffer Range The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffers function through a process of chemical equilibrium. Once this range has been exceeded. For this reason, buffer. Process Buffer Range.
From www.hamiltoncompany.com
DuraCal pH Buffers Buffer Solution pH Hamilton Company Process Buffer Range For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. When you add an. Process Buffer Range.
From biopharma-asia.com
Production of InSpecification Buffer on Demand for Batch Processes Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. Has the largest buffer capacity for its concentration). A weak acid and its conjugate base, or b. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffers function through a process of chemical equilibrium. Once. Process Buffer Range.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Process Buffer Range Once this range has been exceeded. Has the largest buffer capacity for its concentration). Buffers function through a process of chemical equilibrium. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. The buffer range is the range of ph values over which a buffer is most effective (i.e. For this reason, buffer. Process Buffer Range.
From slideplayer.com
Acids, Bases, and the pH scale ppt download Process Buffer Range Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. The buffer range is the range of ph values over which a buffer is most effective (i.e. A weak acid and its conjugate base, or b. For this reason, buffer solutions are used in a wide range of chemical applications, primarily. Process Buffer Range.
From animalia-life.club
Phosphate Buffer System Equation Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffers function through a process of chemical equilibrium. Has the largest buffer capacity for its concentration). For this reason, buffer solutions are used in a wide. Process Buffer Range.
From www.wizeprep.com
Buffer Range Problems Wize University Chemistry Textbook Wizeprep Process Buffer Range Has the largest buffer capacity for its concentration). For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. The buffer. Process Buffer Range.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Process Buffer Range Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Buffers function through a process of chemical equilibrium. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer range can be defined as. Process Buffer Range.
From www.crawfordscientific.com
Buffer choice for HPLC separations Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers function through a process of chemical equilibrium. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. The buffer range can be defined as ± 1 ph unit on either. Process Buffer Range.
From www.cytivalifesciences.com
Inline dilution for chromatography buffer preparation Cytiva Process Buffer Range Buffers function through a process of chemical equilibrium. A weak acid and its conjugate base, or b. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and. Process Buffer Range.
From slideplayer.com
Buffer Effectiveness, Titrations, and pH Curves ppt download Process Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and. Process Buffer Range.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Process Buffer Range Has the largest buffer capacity for its concentration). Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Buffers function through a process of chemical equilibrium. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Buffers are characterized. Process Buffer Range.
From www.chegg.com
Solved Consider the following questions about buffer Process Buffer Range Has the largest buffer capacity for its concentration). The buffer range is the range of ph values over which a buffer is most effective (i.e. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Once this range has been exceeded. Buffers function through a process of chemical equilibrium. Buffers and. Process Buffer Range.
From www.bostonbioproducts.com
Custom Buffer Manufacturing Boston BioProducts Process Buffer Range For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Buffers and how they control hydrogen ion concentrations, a brief. Process Buffer Range.
From www.sartorius.com
Downstream Buffers Sartorius Process Buffer Range Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers function through a. Process Buffer Range.
From dandkmotorsports.com
Hepes Buffer Recipe Ph 7 2 Dandk Organizer Process Buffer Range The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. Has the largest buffer capacity for its concentration). Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Once this range has been. Process Buffer Range.
From www.chegg.com
Solved Having calculated the pKG and buffer ranges for each Process Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Once this range has been exceeded. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. The buffer range. Process Buffer Range.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Buffers are. Process Buffer Range.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Process Buffer Range For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Buffers function through a process of chemical equilibrium. The buffer. Process Buffer Range.
From www.youtube.com
Buffers and Titration Curves YouTube Process Buffer Range Buffers function through a process of chemical equilibrium. The buffer range is the range of ph values over which a buffer is most effective (i.e. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. For this reason, buffer solutions are used in a wide. Process Buffer Range.
From ruslan179144.blogspot.com
Define Definition Of Buffer Capacity Robert Romero Coiffure Process Buffer Range A weak acid and its conjugate base, or b. Buffers function through a process of chemical equilibrium. Once this range has been exceeded. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer range is the range of ph values over which. Process Buffer Range.
From www.alfalaval.in
Alfa Laval Buffer storage Process Buffer Range Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A weak acid and its conjugate base, or b. The buffer range is the range of ph values over which a. Process Buffer Range.
From www.pmknowledgecenter.com
Critical Chain/Buffer Management Sizing project and feeding buffers Process Buffer Range When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Once this range has been exceeded. The buffer range is the range of ph values over which a buffer is most. Process Buffer Range.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Process Buffer Range When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Once this range has been exceeded. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. For this reason, buffer solutions are used in a wide range of. Process Buffer Range.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. A weak acid and its conjugate base, or b. When you add an acid to a buffer, the. Process Buffer Range.
From www.youtube.com
Buffers and pH Biology YouTube Process Buffer Range Buffers function through a process of chemical equilibrium. Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. Has the largest buffer capacity for its concentration). The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed.. Process Buffer Range.
From pubs.rsc.org
(Un)suitability of the use of pH buffers in biological, biochemical and Process Buffer Range Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value (in the production of dyes, in. Has the largest buffer capacity for its concentration). The buffer. Process Buffer Range.
From www.youtube.com
Buffer range and capacity YouTube Process Buffer Range A weak acid and its conjugate base, or b. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. For this reason, buffer solutions are used in a wide range of chemical applications, primarily as reagents that allow to maintain a constant ph value. Process Buffer Range.
From slideplayer.com
principles and modern applications ppt download Process Buffer Range When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Once this range has been exceeded. A weak acid and its conjugate base, or b. Buffers function through a process of chemical equilibrium. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Process Buffer Range.
From www.sartorius.com
Downstream Buffers Sartorius Process Buffer Range The buffer range is the range of ph values over which a buffer is most effective (i.e. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A weak acid and its conjugate base, or b. For this reason, buffer solutions are used in. Process Buffer Range.
From www.researchgate.net
Any suggestion of buffer solution for photocatalysis using ZnO Process Buffer Range The buffer range can be defined as ± 1 ph unit on either side of pka of the acid from which the buffer is formed. Once this range has been exceeded. Has the largest buffer capacity for its concentration). When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffers and how they. Process Buffer Range.