Definition Medical Device Eu . The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european union (eu) they must undergo a conformity. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Medical devices are products or equipment intended for a medical purpose.
from clin-r.com
Medical devices are products or equipment intended for a medical purpose. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions.
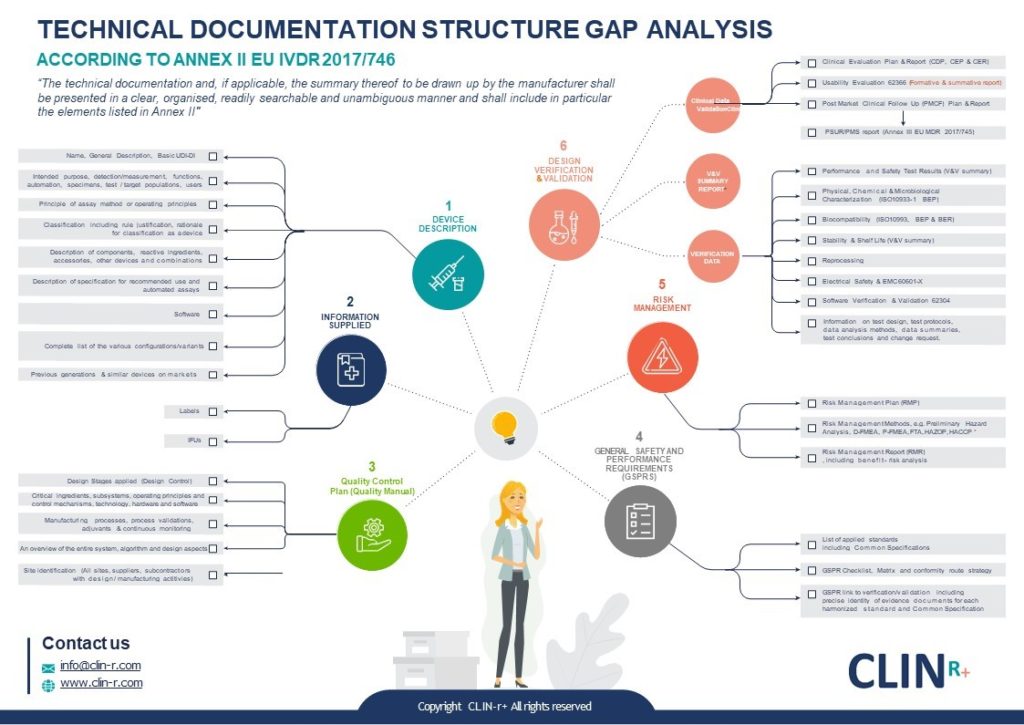
EU MDR how to structure your Medical Device Technical Document Clin R
Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Medical devices are products or equipment intended for a medical purpose.
From www.productcomplianceinstitute.com
EU New Guidance on the definition of medical devices Definition Medical Device Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The complete definition of the term medical device is laid down in article 2 (1). Definition Medical Device Eu.
From operonstrategist.com
Medical Device Classification EU MDR Guide) Operon Strategist Definition Medical Device Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards. Definition Medical Device Eu.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The complete definition of the term medical device is laid down in article 2 (1). Definition Medical Device Eu.
From pepgra.com
Preparing For The Future The New European Union Medical Devices Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity.. Definition Medical Device Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Definition Medical Device Eu The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Regulation (eu) 2017/745 of the. Definition Medical Device Eu.
From omcmedical.com
Exceptional Use of Medical Devices in UK & EU OMC Medical Definition Medical Device Eu A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the. Definition Medical Device Eu.
From www.scribd.com
Medical Devices For The EU 070910 PDF Medical Device European Union Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. In the european union (eu) they must undergo a conformity. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu). Definition Medical Device Eu.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Definition Medical Device Eu In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional. Definition Medical Device Eu.
From easymedicaldevice.com
EU Medical Device Classification Form Medical Device Regulation and Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Publication of regulation (eu) 2023/607 amending. Definition Medical Device Eu.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Definition Medical Device Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. The. Definition Medical Device Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and. Definition Medical Device Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Definition Medical Device Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is any product used. Definition Medical Device Eu.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. (1) ‘medical. Definition Medical Device Eu.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The complete definition of the term medical device is. Definition Medical Device Eu.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Definition Medical Device Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical.. Definition Medical Device Eu.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or. Definition Medical Device Eu.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV Definition Medical Device Eu In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745.. Definition Medical Device Eu.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal. Definition Medical Device Eu.
From www.youtube.com
Classification of Medical Devices EU 2017/745 YouTube Definition Medical Device Eu In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical. Definition Medical Device Eu.
From www.artixio.com
Regulation of Reusable Medical Devices under EU MDR Definition Medical Device Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european. Definition Medical Device Eu.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Definition Medical Device Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Medical devices are products. Definition Medical Device Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Definition Medical Device Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The medical device. Definition Medical Device Eu.
From credevo.com
Europe Medical Device Market Approval Credevo Articles Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Medical devices are products or equipment intended for. Definition Medical Device Eu.
From de.slideshare.net
Medical device definition Definition Medical Device Eu The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european union (eu) they must undergo a conformity. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746. Definition Medical Device Eu.
From www.presentationeze.com
EU Medical Device Classification per the EU Directives PresentationEZE Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. Medical devices are products or equipment intended for a medical purpose. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes. Definition Medical Device Eu.
From medicaldevicehq.com
Design control for medical devices what is it and why you should do it Definition Medical Device Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The complete. Definition Medical Device Eu.
From www.i3cglobal.com
EU Medical Device Classification Examples and Rules Definition Medical Device Eu Medical devices are products or equipment intended for a medical purpose. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or. Definition Medical Device Eu.
From www.greenlight.guru
Medical Device Adverse Event Reporting Regulations EU vs. US Definition Medical Device Eu In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. A medical device is. Definition Medical Device Eu.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Definition Medical Device Eu A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. The. Definition Medical Device Eu.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. In the european union (eu) they must undergo. Definition Medical Device Eu.
From medicaldevicehq.com
Different classifications rules for medical device software An Definition Medical Device Eu The complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Regulation (eu) 2017/745 of the european parliament and. Definition Medical Device Eu.
From pepgra.com
Medical Device Classification In The European Union pepgra Definition Medical Device Eu In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. The complete definition of the term medical device is laid down in article 2 (1). Definition Medical Device Eu.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Definition Medical Device Eu Publication of regulation (eu) 2023/607 amending regulations (eu) 2017/745 and (eu) 2017/746 as regards the transitional provisions. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Medical devices are products or equipment intended for a medical purpose. The medical device regulation (mdr), which was adopted in april 2017, changes the. Definition Medical Device Eu.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Definition Medical Device Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. The complete definition of the term medical device is laid down. Definition Medical Device Eu.
From www2.deloitte.com
The new European Union medical devices regulation Deloitte Life Definition Medical Device Eu (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. Medical devices are products or equipment intended for a medical purpose. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the european union (eu) they must undergo a conformity. A medical device is any product. Definition Medical Device Eu.