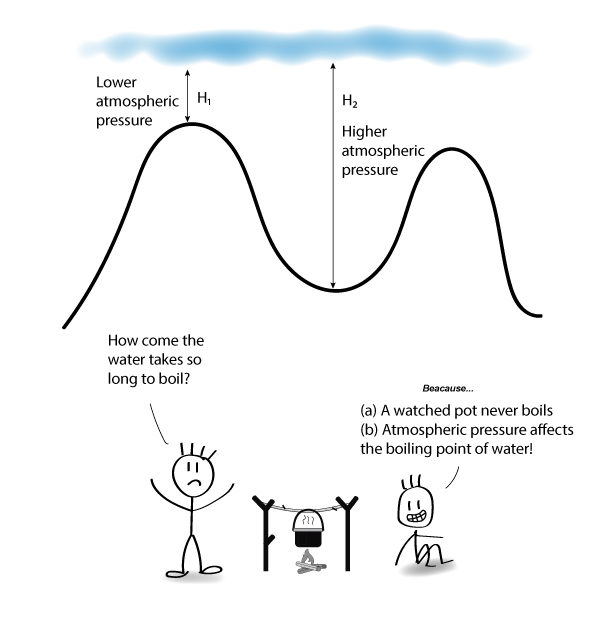
Do Things Boil Faster At Higher Elevations . It seems like one of those basic science facts: It depends on where you’re doing the boiling. More specifically, it affects a very important component of cooking: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. The thing is, if you. Because foods that are boiled are. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The temperature at which water boils decreases as elevation increases. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. More energy, as in higher heat, makes molecules move even faster.
from surfguppy.com
When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It depends on where you’re doing the boiling. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Because foods that are boiled are. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. More specifically, it affects a very important component of cooking: More energy, as in higher heat, makes molecules move even faster. It seems like one of those basic science facts: Water boils at 212 degrees fahrenheit (100 degrees celsius), right?
How does Atmospheric Pressure Affect Boiling Point
Do Things Boil Faster At Higher Elevations More energy, as in higher heat, makes molecules move even faster. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). The temperature at which water boils decreases as elevation increases. It seems like one of those basic science facts: More energy, as in higher heat, makes molecules move even faster. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. More specifically, it affects a very important component of cooking: Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. It depends on where you’re doing the boiling. Because foods that are boiled are.
From slideplayer.com
Colligative Properties. ppt download Do Things Boil Faster At Higher Elevations Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The temperature at which water boils decreases as elevation increases. It depends on where you’re doing the boiling. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you. One definition of boiling point. Do Things Boil Faster At Higher Elevations.
From www.youtube.com
Study Boiling Point of Water with Altitude YouTube Do Things Boil Faster At Higher Elevations The thing is, if you. More energy, as in higher heat, makes molecules move even faster. It depends on where you’re doing the boiling. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It seems like one of those basic science facts: In fact, water will boil at about 202 degrees in. Do Things Boil Faster At Higher Elevations.
From www.gauthmath.com
Solved Why does water vapor condense at higher elevations? faster Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: Because foods that are boiled are. More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will. Do Things Boil Faster At Higher Elevations.
From www.pinterest.com
Why Does Water Boil Faster at Higher Altitude? The voice, Your voice Do Things Boil Faster At Higher Elevations When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. More energy, as in higher heat, makes molecules move even faster. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. One definition of boiling point is the temperature at which. Do Things Boil Faster At Higher Elevations.
From slideplayer.com
Hope You Enjoyed Your Break!! ppt download Do Things Boil Faster At Higher Elevations The thing is, if you. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. The temperature at which water boils decreases as elevation increases. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. More energy, as in higher heat, makes. Do Things Boil Faster At Higher Elevations.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Do Things Boil Faster At Higher Elevations It depends on where you’re doing the boiling. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It seems like one of those basic science facts: One definition of boiling point. Do Things Boil Faster At Higher Elevations.
From mycurrentelevation.org
Boiling Point at Elevation & Altitude Calculator My Current Elevation Do Things Boil Faster At Higher Elevations It seems like one of those basic science facts: More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The thing is, if you. One definition of boiling point is the. Do Things Boil Faster At Higher Elevations.
From www.slideserve.com
PPT Properties of Liquids and Solids PowerPoint Presentation, free Do Things Boil Faster At Higher Elevations Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It depends on where you’re doing the boiling. The thing is, if you. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. It seems like one of those basic science facts: When atmospheric pressure is lower, such as at a higher. Do Things Boil Faster At Higher Elevations.
From gioixxrqs.blob.core.windows.net
Do Things Bake Faster At High Altitude at Mathew James blog Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science facts: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Because foods that are boiled are. More energy, as in higher heat,. Do Things Boil Faster At Higher Elevations.
From exochgnhh.blob.core.windows.net
Why Do Things Expand At High Altitudes at Jackie Pruneda blog Do Things Boil Faster At Higher Elevations When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The thing is, if you. More specifically, it affects a very important. Do Things Boil Faster At Higher Elevations.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Because foods that are boiled are. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). Less energy means less heat, which means water will boil at a lower. Do Things Boil Faster At Higher Elevations.
From www.tastingtable.com
How To Adjust Your Water Boiling Process For High Altitudes Do Things Boil Faster At Higher Elevations Because foods that are boiled are. More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The temperature at which. Do Things Boil Faster At Higher Elevations.
From exogrqaue.blob.core.windows.net
Time Goes Faster At Higher Altitudes at Dennis Townsend blog Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. More specifically, it affects a very important component of cooking: Because foods that are boiled are. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Water boils at 212 degrees. Do Things Boil Faster At Higher Elevations.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Do Things Boil Faster At Higher Elevations When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It depends on where you’re doing the boiling. More energy, as in higher heat, makes molecules move even faster. More specifically, it affects a very important component of cooking: The temperature at which water boils decreases as elevation increases. The thing is, if. Do Things Boil Faster At Higher Elevations.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Do Things Boil Faster At Higher Elevations Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When you boil water, you're literally speeding. Do Things Boil Faster At Higher Elevations.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It depends on where you’re doing the boiling. Because foods that are boiled are. Less energy means. Do Things Boil Faster At Higher Elevations.
From slideplayer.com
Solutions Unit. ppt download Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It depends on where you’re doing the boiling. More energy, as in higher heat, makes molecules move even faster. One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble).. Do Things Boil Faster At Higher Elevations.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: The temperature at which water boils decreases as elevation increases. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It depends on where you’re doing the boiling. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When. Do Things Boil Faster At Higher Elevations.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. More specifically, it affects a very important component of cooking: One definition of boiling point is the temperature at which the. Do Things Boil Faster At Higher Elevations.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When you boil water, you're literally speeding. Do Things Boil Faster At Higher Elevations.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Do Things Boil Faster At Higher Elevations It seems like one of those basic science facts: More specifically, it affects a very important component of cooking: More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. It depends on where you’re doing the boiling. One definition of boiling. Do Things Boil Faster At Higher Elevations.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. Because foods that are boiled are. The temperature at which water boils decreases as elevation increases. It seems like one of those basic science facts: One definition of boiling point is the. Do Things Boil Faster At Higher Elevations.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Do Things Boil Faster At Higher Elevations The thing is, if you. More specifically, it affects a very important component of cooking: One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). It seems like one of those basic science facts: More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which. Do Things Boil Faster At Higher Elevations.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Do Things Boil Faster At Higher Elevations Because foods that are boiled are. It depends on where you’re doing the boiling. More energy, as in higher heat, makes molecules move even faster. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? More specifically, it affects a very important component of cooking: One definition of boiling point is the temperature at which the substance's vapour pressure (the. Do Things Boil Faster At Higher Elevations.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Do Things Boil Faster At Higher Elevations Water boils at 212 degrees fahrenheit (100 degrees celsius), right? More specifically, it affects a very important component of cooking: The temperature at which water boils decreases as elevation increases. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It depends on where you’re doing the boiling. Because foods that are boiled. Do Things Boil Faster At Higher Elevations.
From gioixxrqs.blob.core.windows.net
Do Things Bake Faster At High Altitude at Mathew James blog Do Things Boil Faster At Higher Elevations The temperature at which water boils decreases as elevation increases. Because foods that are boiled are. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. More specifically, it affects a very important component of cooking: It depends on where you’re doing the boiling. One definition of boiling point is the temperature. Do Things Boil Faster At Higher Elevations.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Do Things Boil Faster At Higher Elevations The temperature at which water boils decreases as elevation increases. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. More specifically, it affects a very important component of cooking: Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It depends on where you’re doing the boiling. When atmospheric pressure is lower,. Do Things Boil Faster At Higher Elevations.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Do Things Boil Faster At Higher Elevations It depends on where you’re doing the boiling. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you. Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. It seems like one of those basic. Do Things Boil Faster At Higher Elevations.
From facts.net
20 Fascinating Facts About Boiling Point Elevation Do Things Boil Faster At Higher Elevations One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). More specifically, it affects a very important component of cooking: It depends on where you’re doing the boiling. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When. Do Things Boil Faster At Higher Elevations.
From www.kindpng.com
Peppa Pig Banned Does Water Boil Faster At Higher Altitudes, HD Png Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: More energy, as in higher heat, makes molecules move even faster. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water. Do Things Boil Faster At Higher Elevations.
From facts.net
20 Fascinating Facts About Boiling Point Do Things Boil Faster At Higher Elevations When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The temperature at which water boils decreases as elevation increases. Because foods that are boiled are. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Less. Do Things Boil Faster At Higher Elevations.
From slideplayer.com
Intermolecular Forces ppt download Do Things Boil Faster At Higher Elevations The thing is, if you. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Because foods that are boiled are. It depends on where you’re doing. Do Things Boil Faster At Higher Elevations.
From fyoxouusm.blob.core.windows.net
Does It Take Water Longer To Boil In High Altitude at Joseph May blog Do Things Boil Faster At Higher Elevations More specifically, it affects a very important component of cooking: One definition of boiling point is the temperature at which the substance's vapour pressure (the pressure inside the bubble). It seems like one of those basic science facts: Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. In fact, water will. Do Things Boil Faster At Higher Elevations.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Do Things Boil Faster At Higher Elevations Less energy means less heat, which means water will boil at a lower temperature at a higher altitude. More energy, as in higher heat, makes molecules move even faster. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? More specifically, it affects a very important component of cooking: It seems like one of those basic science facts: In fact,. Do Things Boil Faster At Higher Elevations.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Do Things Boil Faster At Higher Elevations It depends on where you’re doing the boiling. More energy, as in higher heat, makes molecules move even faster. Because foods that are boiled are. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. The thing is, if you. The temperature at which water boils decreases as. Do Things Boil Faster At Higher Elevations.