Relation Between Evaporation And Vapour Pressure . The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. It also looks at how saturated vapour pressure. If we have an open container of. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature.
from pediaa.com
If we have an open container of. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. It also looks at how saturated vapour pressure. Therefore it isn't at equilibrium, and the pan of water quickly evaporates.
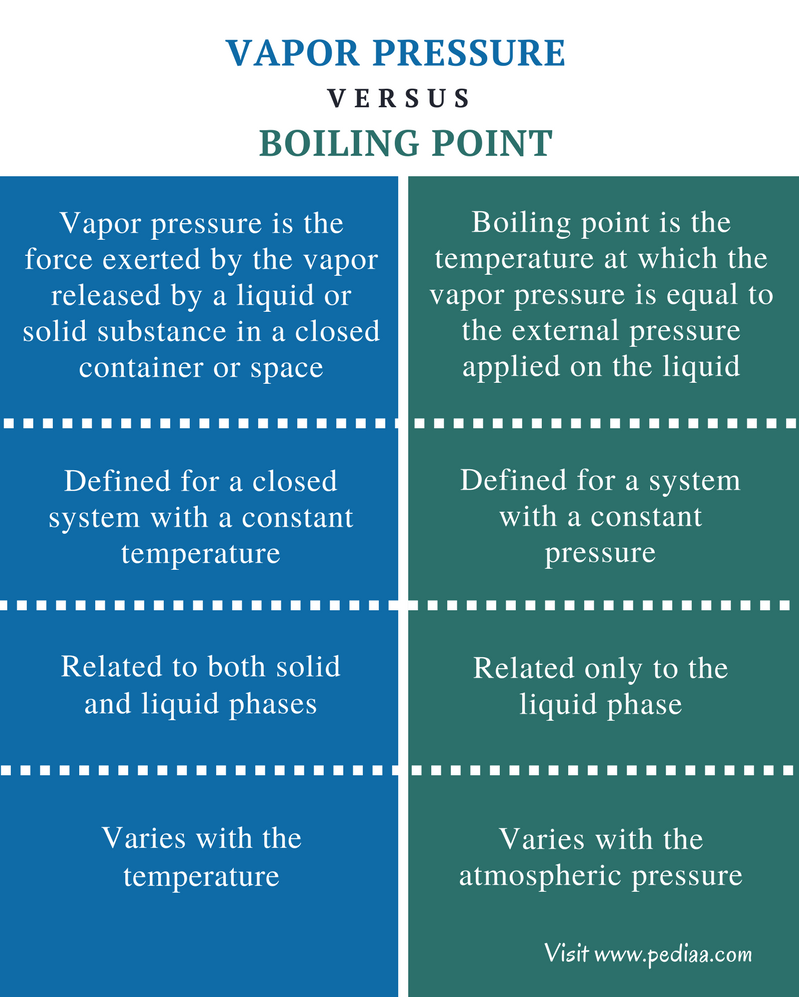
Difference Between Vapor Pressure and Boiling Point Definition
Relation Between Evaporation And Vapour Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. If we have an open container of. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. It also looks at how saturated vapour pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Therefore it isn't at equilibrium, and the pan of water quickly evaporates. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the.
From saylordotorg.github.io
Vapor Pressure Relation Between Evaporation And Vapour Pressure Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. If we have an open container of. If the partial pressure is less than the vapor pressure, then evaporation will take place,. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Relation Between Evaporation And Vapour Pressure If we have an open container of. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. At 100% humidity, the partial pressure is. Relation Between Evaporation And Vapour Pressure.
From saylordotorg.github.io
Vapor Pressure Relation Between Evaporation And Vapour Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Relation Between Evaporation And Vapour Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Relation Between Evaporation And Vapour Pressure It also looks at how saturated vapour pressure. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. If we. Relation Between Evaporation And Vapour Pressure.
From sciencenotes.org
Clausius Clapeyron Equation Relation Between Evaporation And Vapour Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It also looks at how saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Relation Between Evaporation And Vapour Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. If we have an. Relation Between Evaporation And Vapour Pressure.
From socratic.org
Explain how each of the following affects the vapour pressure of a Relation Between Evaporation And Vapour Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the.. Relation Between Evaporation And Vapour Pressure.
From socratic.org
What is the relation between critical temperature and boiling point or Relation Between Evaporation And Vapour Pressure This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. If we have an open container of. Vapor pressure is defined as the partial. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Degree of Dissociation . Relation between vapor density & Degree of Relation Between Evaporation And Vapour Pressure If we have an open container of. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. It also looks at how saturated vapour pressure. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Generally a substance's vapor pressure increases as. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT AP Chapter 11 PowerPoint Presentation, free download ID2275798 Relation Between Evaporation And Vapour Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Vapor pressure is. Relation Between Evaporation And Vapour Pressure.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Relation Between Evaporation And Vapour Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. We can express the nonlinear relationship between vapor pressure. Relation Between Evaporation And Vapour Pressure.
From www.vrogue.co
The Saturated Water Vapor Pressure Curve With Marked vrogue.co Relation Between Evaporation And Vapour Pressure If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. If we have an open container of. At 100% humidity, the partial pressure is equal to the vapor pressure,. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Relation Between Evaporation And Vapour Pressure Therefore it isn't at equilibrium, and the pan of water quickly evaporates. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. It also looks at how saturated vapour pressure. At 100%. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Relation Between Evaporation And Vapour Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. That is,. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Raoults Law and Vapor Pressure Chemistry Tutorial YouTube Relation Between Evaporation And Vapour Pressure This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. It also looks at how saturated vapour pressure. If we have an open container of. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. Even though the. Relation Between Evaporation And Vapour Pressure.
From byjus.com
what is the differance between partial pressure and vapour pressure? Relation Between Evaporation And Vapour Pressure At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Therefore it isn't at equilibrium, and. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Moisture Relationships PowerPoint Presentation, free download Relation Between Evaporation And Vapour Pressure It also looks at how saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. This page. Relation Between Evaporation And Vapour Pressure.
From mavink.com
Vapor Pressure And Boiling Point Relationship Relation Between Evaporation And Vapour Pressure If we have an open container of. It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Generally a. Relation Between Evaporation And Vapour Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Relation Between Evaporation And Vapour Pressure It also looks at how saturated vapour pressure. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. If we have an open container of. Therefore it isn't. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Relation Between Evaporation And Vapour Pressure At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. This page looks at. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Vapor density, YouTube Relation Between Evaporation And Vapour Pressure If we have an open container of. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. It also looks at how saturated vapour pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Therefore it isn't at equilibrium, and the pan of water quickly evaporates.. Relation Between Evaporation And Vapour Pressure.
From vacaero.com
Evaporation Relation Between Evaporation And Vapour Pressure We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); It also looks at how saturated vapour pressure. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Relation Between Evaporation And Vapour Pressure This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. If we have an open container of. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. It also looks at how saturated vapour. Relation Between Evaporation And Vapour Pressure.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Relation Between Evaporation And Vapour Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. We can express the. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID Relation Between Evaporation And Vapour Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the.. Relation Between Evaporation And Vapour Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Relation Between Evaporation And Vapour Pressure It also looks at how saturated vapour pressure. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. At 100% humidity, the partial pressure is equal to the vapor pressure, and no. Relation Between Evaporation And Vapour Pressure.
From byjus.com
18. What is the relation between osmotic pressure (π) and relative Relation Between Evaporation And Vapour Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Vapor pressure is defined. Relation Between Evaporation And Vapour Pressure.
From www.researchgate.net
Relationship between the saturated vapor pressure and temperature of Relation Between Evaporation And Vapour Pressure If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases. Relation Between Evaporation And Vapour Pressure.
From www.researchgate.net
A. Relationship between vapor pressure, relative humidity and Relation Between Evaporation And Vapour Pressure It also looks at how saturated vapour pressure. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. The vapor pressure of a liquid is the equilibrium pressure of. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor Relation Between Evaporation And Vapour Pressure Therefore it isn't at equilibrium, and the pan of water quickly evaporates. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If we have an open container of. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Vapor pressure is defined as. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Relation Between Evaporation And Vapour Pressure That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. The vapor pressure of a liquid is the equilibrium pressure of a. Relation Between Evaporation And Vapour Pressure.
From byjus.com
Briefly explain Derivation of relative lowering of vapour pressure. Relation Between Evaporation And Vapour Pressure If we have an open container of. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. This page looks at how the equilibrium between a liquid (or a solid) and its vapour leads to the idea of a saturated vapour pressure. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water. Relation Between Evaporation And Vapour Pressure.
From www.slideserve.com
PPT Vapour Pressure and Heat PowerPoint Presentation, free download Relation Between Evaporation And Vapour Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. It. Relation Between Evaporation And Vapour Pressure.
From www.youtube.com
Difference Between Partial Vapour Pressure And Pressure What is Relation Between Evaporation And Vapour Pressure Even though the water vapor pressure (even in the sahara!) is nonzero, it is way less than saturated. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. It also looks at how saturated. Relation Between Evaporation And Vapour Pressure.
From pharmacyscope.com
Vapour pressure Pharmacy Scope Relation Between Evaporation And Vapour Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. It also looks at how saturated vapour pressure. Therefore it isn't at equilibrium, and the pan of water quickly evaporates. If the. Relation Between Evaporation And Vapour Pressure.