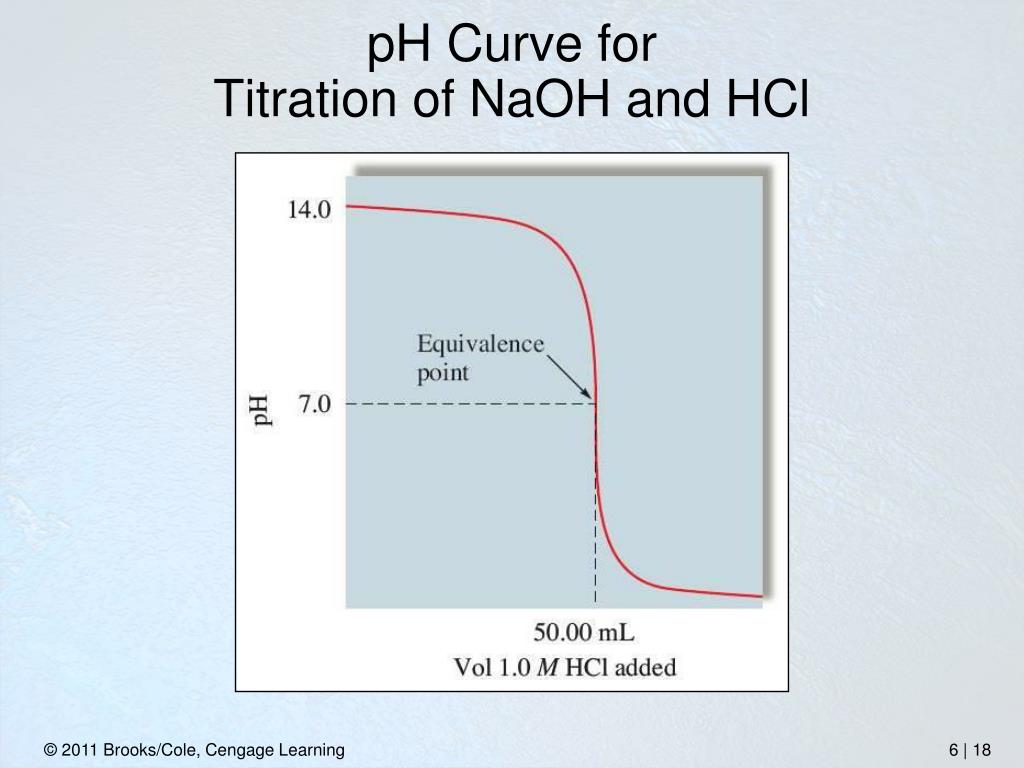
Hcl And Naoh Titration Indicator . The ph ranges over which two common In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The initial volume and final volume of all three trails were subtracted to find. titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation. volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). To determine the composition of the.
from mungfali.com
To determine the composition of the. volume measurements play a key role in titration. The ph ranges over which two common titrating sodium hydroxide with hydrochloric acid. The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
HCl NaOH Titration
Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find. To determine the composition of the. The ph ranges over which two common In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). volume measurements play a key role in titration. titrating sodium hydroxide with hydrochloric acid.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Hcl And Naoh Titration Indicator To determine the composition of the. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). titrating sodium hydroxide with hydrochloric acid. The ph ranges over which two common In this. Hcl And Naoh Titration Indicator.
From snipe.fm
️ Standardization of hcl and naoh. Titrating sodium hydroxide with Hcl And Naoh Titration Indicator In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The initial volume and final volume of all three trails were subtracted to find. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid. Hcl And Naoh Titration Indicator.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Indicator The ph ranges over which two common volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of. Hcl And Naoh Titration Indicator.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). volume. Hcl And Naoh Titration Indicator.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Hcl And Naoh Titration Indicator In association with nuffield foundation. volume measurements play a key role in titration. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). titrating sodium hydroxide with hydrochloric acid. The. Hcl And Naoh Titration Indicator.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Hcl And Naoh Titration Indicator The ph ranges over which two common volume measurements play a key role in titration. titrating sodium hydroxide with hydrochloric acid. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. Hcl And Naoh Titration Indicator.
From cetdge.blogspot.com
Best Indicator For Hcl And Naoh Titration CETDGE Hcl And Naoh Titration Indicator The ph ranges over which two common this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). The initial volume and final volume of all three trails were subtracted to find. They. Hcl And Naoh Titration Indicator.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Hcl And Naoh Titration Indicator.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Hcl And Naoh Titration Indicator To determine the composition of the. titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation. volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Hcl And Naoh Titration Indicator.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To determine the composition of the. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl). Hcl And Naoh Titration Indicator.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. volume measurements play a key role in titration. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100. Hcl And Naoh Titration Indicator.
From chart-studio.plotly.com
Titration Curve HCLH3PO4 with NaOH scatter chart made by Meerika Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. To determine the composition of the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl). Hcl And Naoh Titration Indicator.
From www.youtube.com
HClNaOH Titration (indicator) BTEC Level 3 Applied Science Hcl And Naoh Titration Indicator The initial volume and final volume of all three trails were subtracted to find. titrating sodium hydroxide with hydrochloric acid. To determine the composition of the. volume measurements play a key role in titration. The ph ranges over which two common this figure shows plots of ph versus volume of base added for the titration of 50.0. Hcl And Naoh Titration Indicator.
From www.studypool.com
SOLUTION Titration with hcl and naoh Studypool Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. titrating sodium hydroxide with hydrochloric acid. To determine the composition of the. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid. Hcl And Naoh Titration Indicator.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The ph ranges over which two common The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. volume measurements play a key role in titration. In this experiment. Hcl And Naoh Titration Indicator.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Hcl And Naoh Titration Indicator The ph ranges over which two common titrating sodium hydroxide with hydrochloric acid. In association with nuffield foundation. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium. Hcl And Naoh Titration Indicator.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Hcl And Naoh Titration Indicator this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). To determine the composition of the. In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. In this experiment students. Hcl And Naoh Titration Indicator.
From www.studocu.com
Titration of HCl with Na OH C12510 Titration of HCl with NaOH C125 Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of. Hcl And Naoh Titration Indicator.
From www.youtube.com
Titration of HCl with NaOH YouTube Hcl And Naoh Titration Indicator The ph ranges over which two common In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find. this figure shows plots of ph versus volume of base added. Hcl And Naoh Titration Indicator.
From mungfali.com
Titration Curve HCl And NaOH Hcl And Naoh Titration Indicator In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The ph ranges over which two common The initial volume and final volume of all three trails were subtracted to find. titrating sodium hydroxide with hydrochloric acid. To determine the composition of the. volume measurements play a key role. Hcl And Naoh Titration Indicator.
From www.youtube.com
NaOH vs HCl Titration using Phenolphthalein Class 11 Chemistry Lab Hcl And Naoh Titration Indicator The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). They then concentrate. Hcl And Naoh Titration Indicator.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Hcl And Naoh Titration Indicator volume measurements play a key role in titration. titrating sodium hydroxide with hydrochloric acid. The ph ranges over which two common The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml. Hcl And Naoh Titration Indicator.
From mungfali.com
Titration Curve HCl And NaOH Hcl And Naoh Titration Indicator volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In association with nuffield foundation. The ph ranges over which two common titrating sodium hydroxide with. Hcl And Naoh Titration Indicator.
From byjus.com
The graph of pH during the titration of NaOH and HCl Hcl And Naoh Titration Indicator volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m. Hcl And Naoh Titration Indicator.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Hcl And Naoh Titration Indicator The ph ranges over which two common In association with nuffield foundation. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The initial volume and final volume of all three trails were subtracted to find. this figure shows plots of ph versus volume of base added for the titration. Hcl And Naoh Titration Indicator.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Hcl And Naoh Titration Indicator The initial volume and final volume of all three trails were subtracted to find. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The ph ranges over which two common volume measurements play a key role in titration. this figure shows plots of ph versus volume of base. Hcl And Naoh Titration Indicator.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Hcl And Naoh Titration Indicator In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To determine the composition of the. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The ph ranges over which two common volume measurements play a key role in titration. The initial volume and. Hcl And Naoh Titration Indicator.
From mungfali.com
HCl NaOH Titration Hcl And Naoh Titration Indicator The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. volume measurements play a key role in titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The ph ranges over which two. Hcl And Naoh Titration Indicator.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Hcl And Naoh Titration Indicator To determine the composition of the. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. volume measurements play a key role in titration. The initial volume and final volume of all three trails were subtracted to find.. Hcl And Naoh Titration Indicator.
From cma-science.nl
» HCl and NaOH Titration Simulation CMA Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. titrating sodium hydroxide with hydrochloric acid. The initial volume and final volume of all three trails were subtracted to find. The ph ranges over which two common In association with nuffield foundation. this figure shows plots of ph versus volume of base added. Hcl And Naoh Titration Indicator.
From www.studypool.com
SOLUTION Laboratory Report Standardization of NaOH with KHP and Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. volume measurements play a key role in titration. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). In association with nuffield foundation. The. Hcl And Naoh Titration Indicator.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Hcl And Naoh Titration Indicator titrating sodium hydroxide with hydrochloric acid. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Hcl And Naoh Titration Indicator.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. titrating sodium hydroxide with hydrochloric acid. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m \(naoh\).. Hcl And Naoh Titration Indicator.
From www.youtube.com
DOUBLE INDICATOR TITRATION part 01Mixture of Sodium Carbonate and Hcl And Naoh Titration Indicator To determine the composition of the. The ph ranges over which two common The initial volume and final volume of all three trails were subtracted to find. In association with nuffield foundation. titrating sodium hydroxide with hydrochloric acid. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. volume measurements play a key. Hcl And Naoh Titration Indicator.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Hcl And Naoh Titration Indicator They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. volume measurements play a key role in titration. To determine the composition of the. The ph ranges over which two common In association with nuffield foundation. this figure shows plots of ph versus volume of base added for the titration of 50.0 ml. Hcl And Naoh Titration Indicator.