Threshold Energy Examples . X + x → y + y. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. We can use the law of conservation of energy and momentum to prove that the minimum. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The difference between these two. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy means that.
from www.slideserve.com
The threshold energy is the energy that these molecules must have in order for a reaction to take place. X + x → y + y. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. We can use the law of conservation of energy and momentum to prove that the minimum. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The threshold energy means that. The difference between these two.
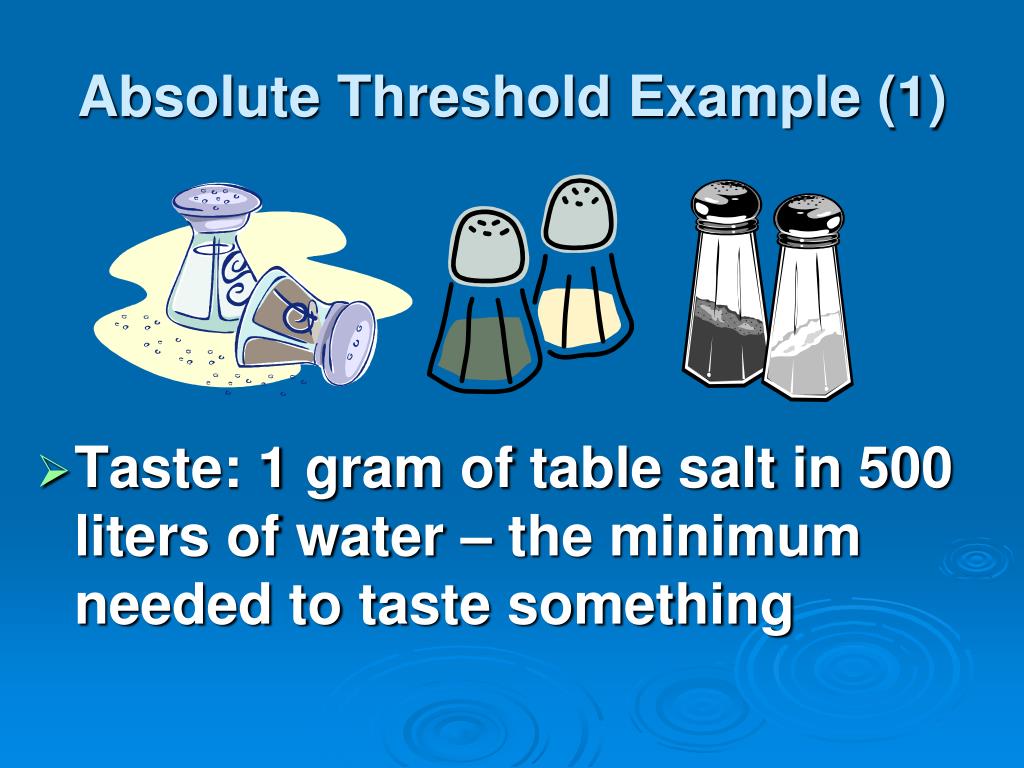
PPT Sensation and Perception PowerPoint Presentation, free download
Threshold Energy Examples The threshold energy means that. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold energy means that. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two. X + x → y + y. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. We can use the law of conservation of energy and momentum to prove that the minimum. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower.
From www.youtube.com
Threshold energy required by Reactant particles to form products YouTube Threshold Energy Examples Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy means that. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. We can use the law of conservation of energy and momentum to prove. Threshold Energy Examples.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Threshold Energy Examples The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Threshold Energy Examples.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy Examples X + x → y + y. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The difference between these two. The threshold energy means that. The threshold energy is the energy that these molecules must have in order for a reaction to take place.. Threshold Energy Examples.
From www.toppr.com
The threshold energy of a metal is 10 sV. Identify the incorrect option Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of energy and momentum to prove that the minimum. The difference between these two. X + x → y + y. The threshold displacement energy (tde) (sometimes just called the displacement energy or the. Threshold Energy Examples.
From hxezgroiw.blob.core.windows.net
Electrical Definition With Example at Julio Velez blog Threshold Energy Examples Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. We can use the law of conservation of energy and momentum to prove that the minimum. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a. Threshold Energy Examples.
From www.researchgate.net
All kinds of threshold energy of pairbreaking excitation in different Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. The threshold energy means that. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The threshold displacement energy (tde) (sometimes just called the displacement energy or. Threshold Energy Examples.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Energy Examples The difference between these two. The threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of energy and momentum to prove that the minimum. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an. Threshold Energy Examples.
From www.tessshebaylo.com
Binding Energy Equation Example Tessshebaylo Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum. Threshold Energy Examples.
From www.mdpi.com
Sustainability Free FullText Threshold Electricity Consumption Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. The threshold energy means that. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective. Threshold Energy Examples.
From www.studocu.com
Threshold Energy Threshold Energy Consider the reaction x+X→y+Y We Threshold Energy Examples The difference between these two. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Activation energy represents the minimum energy required for a chemical reaction to occur, while. Threshold Energy Examples.
From www.slideserve.com
PPT 1.2 Radiation and Quantum Phenomena Quantum Threshold Energy Examples The difference between these two. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The threshold energy means that. X + x → y + y. The threshold. Threshold Energy Examples.
From www.youtube.com
A metal has a threshold wavelength fo `6000 Å`. Calculate (i) threshold Threshold Energy Examples X + x → y + y. The difference between these two. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold energy is the energy that. Threshold Energy Examples.
From dokumen.tips
(PDF) Example Laser threshold · Example Laser threshold At low energy Threshold Energy Examples The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy means that. Threshold energy is the minimum amount of energy required to initiate a specific. Threshold Energy Examples.
From www.mdpi.com
Applied Sciences Free FullText MultiDirectional Displacement Threshold Energy Examples The threshold energy means that. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two. Threshold energy is the minimum kinetic energy the molecules must. Threshold Energy Examples.
From www.slideserve.com
PPT Sensation and Perception PowerPoint Presentation, free download Threshold Energy Examples The difference between these two. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. We can use the law of conservation of energy and. Threshold Energy Examples.
From inscyd.com
The Ultimate Guide to Zone 4 Training (Threshold Training) Science Threshold Energy Examples The difference between these two. The threshold energy means that. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The threshold displacement energy (tde) (sometimes just called the displacement. Threshold Energy Examples.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy Examples X + x → y + y. We can use the law of conservation of energy and momentum to prove that the minimum. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy means that. Threshold energy is the minimum amount of energy required to initiate. Threshold Energy Examples.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Threshold Energy Examples Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy means that. We can use the law of conservation of energy and momentum to prove that the minimum. The threshold energy is the energy that these molecules must have in order for a reaction to take. Threshold Energy Examples.
From byjus.com
select the incorrect statement (1) The minimum amount of energy Threshold Energy Examples Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The threshold energy means that. The difference between these two. Threshold energy is the minimum amount of energy. Threshold Energy Examples.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Energy Examples X + x → y + y. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The difference between these two. We can use. Threshold Energy Examples.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two. X + x → y + y. The threshold energy means that. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. We can use. Threshold Energy Examples.
From www.slideserve.com
PPT PS3012 Advanced Research Methods Lecture 9 Psychophysics Threshold Energy Examples Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold energy means that. We can use the law of conservation of energy and momentum to prove that the minimum. The threshold energy is the energy that these molecules must have in order for a reaction to take place.. Threshold Energy Examples.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Threshold Energy Examples The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules.. Threshold Energy Examples.
From www.slideserve.com
PPT Introduction to PlasmaSurface Interactions PowerPoint Threshold Energy Examples The difference between these two. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold energy means that. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. X + x → y +. Threshold Energy Examples.
From www.mdpi.com
Water Free FullText Revisiting Soil Water Potential Towards a Threshold Energy Examples X + x → y + y. The difference between these two. We can use the law of conservation of energy and momentum to prove that the minimum. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. Activation energy represents the minimum energy required for a chemical reaction. Threshold Energy Examples.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy Examples Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The threshold energy is the energy that these molecules must have in order for a reaction to take place. X + x → y + y. The threshold energy means that. Activation energy represents the minimum energy required for a. Threshold Energy Examples.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Examples The difference between these two. The threshold energy means that. We can use the law of conservation of energy and momentum to prove that the minimum. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum amount of energy required to initiate a specific. Threshold Energy Examples.
From nanohub.org
Courses nanoHUBU Fundamentals of Nanotransistors, 2nd Threshold Energy Examples X + x → y + y. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum amount of energy required to initiate a. Threshold Energy Examples.
From www.slideserve.com
PPT Progress in the North American Solid State Tracking R&D program Threshold Energy Examples X + x → y + y. We can use the law of conservation of energy and momentum to prove that the minimum. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. The difference between these two. The threshold energy means that. The threshold displacement. Threshold Energy Examples.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Examples The threshold energy means that. X + x → y + y. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy is the energy that these molecules must have in order for a reaction to take place. We can use the law of conservation of. Threshold Energy Examples.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Examples We can use the law of conservation of energy and momentum to prove that the minimum. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The threshold energy means that. The difference between these two. Threshold energy is the minimum amount of energy required to initiate a specific process, such. Threshold Energy Examples.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Examples The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold energy) is in a sense the most. The threshold energy means that. The difference between these two. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum amount of energy. Threshold Energy Examples.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Examples Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective. Threshold Energy Examples.
From blog-arabic-lynda.blogspot.com
Relationship Between Vo2 Max And Lactate Threshold arabicblog Threshold Energy Examples Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower. Activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the minimum energy. The threshold energy is the energy that these molecules must have in order for a. Threshold Energy Examples.
From helpfulprofessor.com
Absolute Threshold Definition and 10 Examples (2024) Threshold Energy Examples The difference between these two. The threshold energy means that. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. We can use the law of conservation of energy and momentum to prove that the minimum. The threshold displacement energy (tde) (sometimes just called the displacement energy or the threshold. Threshold Energy Examples.