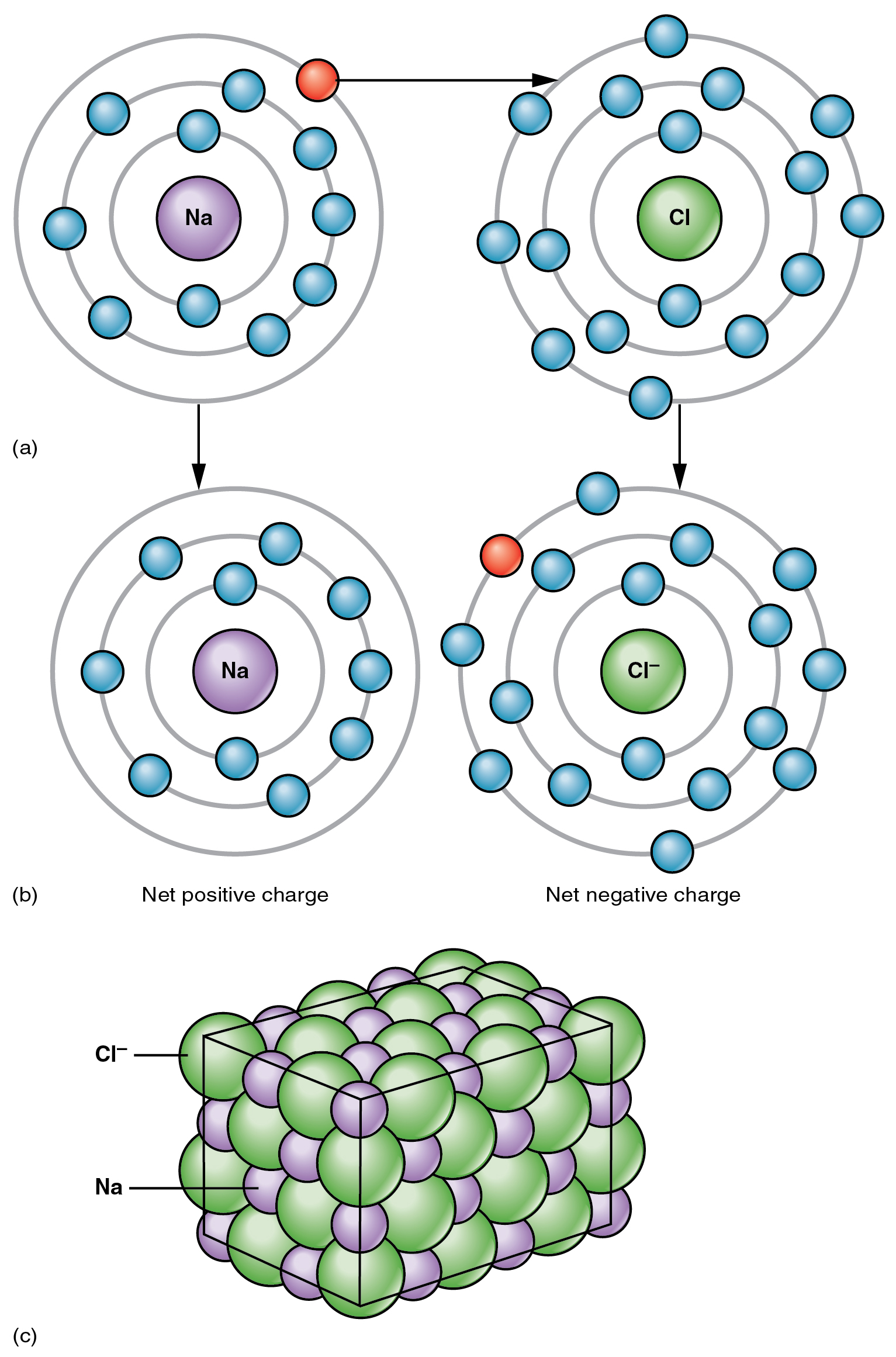
Valence Electrons Does Chlorine Ion . One with two 1s electrons, one with two 2s electrons and six 2p electrons,. (in table salt, this electron comes from the sodium atom.) e− + cl. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. There are one chlorine atom and three. Chlorine has 7 valence electrons. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells:
from philschatz.com
The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. There are one chlorine atom and three.
Chemical Bonds · Anatomy and Physiology
Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. There are one chlorine atom and three. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. (in table salt, this electron comes from the sodium atom.) e− + cl. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine has 7 valence electrons. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. One with two 1s electrons, one with two 2s electrons and six 2p electrons,.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Chlorine has 7 valence electrons. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. There are. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does COCl2 (phosgene) Have? Valence Electrons Does Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only one more electron is needed to achieve an octet in chlorine’s valence shell. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells:. Valence Electrons Does Chlorine Ion.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Valence Electrons Does Chlorine Ion The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. There are one chlorine atom and three. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. As another example, an element like chlorine. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum (Al) Have? Valence Electrons Does Chlorine Ion As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: Chlorine has 7 valence electrons. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. Therefore, the electron configuration of chlorine. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. (in table salt, this electron comes from the sodium atom.) e− + cl. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. As another example, an. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. There are one. Valence Electrons Does Chlorine Ion.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Valence Electrons Does Chlorine Ion The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: Chlorine has 7 valence electrons. Only. Valence Electrons Does Chlorine Ion.
From download4update.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Valence Electrons Does Chlorine Ion Only one more electron is needed to achieve an octet in chlorine’s valence shell. There are one chlorine atom and three. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. 93 rows you may assume the valences of the chemical elements—the. Valence Electrons Does Chlorine Ion.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Valence Electrons Does Chlorine Ion There are one chlorine atom and three. Chlorine has 7 valence electrons. (in table salt, this electron comes from the sodium atom.) e− + cl. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence Electrons Does Chlorine Ion.
From www.youtube.com
How many valence electrons does chlorine have?How to find the valence Valence Electrons Does Chlorine Ion The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Valence Electrons Does Chlorine Ion.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Valence Electrons Does Chlorine Ion Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. Chlorine has 7 valence electrons. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One with. Valence Electrons Does Chlorine Ion.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One with two 1s electrons, one with two 2s electrons and six 2p electrons,. There are one chlorine atom and three. Only one more electron is needed to achieve an octet in chlorine’s. Valence Electrons Does Chlorine Ion.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine. Valence Electrons Does Chlorine Ion.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Valence Electrons Does Chlorine Ion There are one chlorine atom and three. (in table salt, this electron comes from the sodium atom.) e− + cl. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of. Valence Electrons Does Chlorine Ion.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Valence Electrons Does Chlorine Ion As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: There are one chlorine atom and three. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. 93 rows you may assume the valences of the chemical elements—the number of. Valence Electrons Does Chlorine Ion.
From www.crowlex.com
How Many Valence Electrons Does Chlorine Have Crowlex Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of chlorine refers to the arrangement. Valence Electrons Does Chlorine Ion.
From www.youtube.com
Chlorine Electron Configuration YouTube Valence Electrons Does Chlorine Ion Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. Only one more electron is needed to achieve an octet in chlorine’s valence shell. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: The electron configuration of chlorine is. Valence Electrons Does Chlorine Ion.
From famousparenting.com
How Many Valence Electrons Does Chlorine Have Famous Parenting Valence Electrons Does Chlorine Ion One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion.. Valence Electrons Does Chlorine Ion.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Valence Electrons Does Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. (in table salt, this electron comes from the sodium atom.) e− + cl. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion.. Valence Electrons Does Chlorine Ion.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. There are one chlorine atom and three. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. (in table salt, this electron comes from the sodium atom.) e− + cl. The electron configuration of chlorine is 1s22s22p63s23p5 or. Valence Electrons Does Chlorine Ion.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Valence Electrons Does Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. There are one chlorine atom and three. One with two 1s electrons, one. Valence Electrons Does Chlorine Ion.
From ar.inspiredpencil.com
Electron Configuration For Chlorine Valence Electrons Does Chlorine Ion There are one chlorine atom and three. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron. Valence Electrons Does Chlorine Ion.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Valence Electrons Does Chlorine Ion The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. Chlorine has. Valence Electrons Does Chlorine Ion.
From astonishingceiyrs.blogspot.com
Cl Valence Electrons astonishingceiyrs Valence Electrons Does Chlorine Ion There are one chlorine atom and three. Therefore, the electron configuration of chlorine will be 1s2 2s2 2p6 3s2 3p5. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Chlorine has 7 valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The electron configuration of chlorine refers to the arrangement of electrons in. Valence Electrons Does Chlorine Ion.
From www.expii.com
Ions — Definition & Overview Expii Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. There are one chlorine atom and three. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. One with two 1s electrons, one with two. Valence Electrons Does Chlorine Ion.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Valence Electrons Does Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. (in table salt, this electron comes from the sodium atom.) e− + cl. Chlorine has 7 valence electrons. There are one chlorine atom and three. One with two 1s electrons, one with two 2s electrons and. Valence Electrons Does Chlorine Ion.
From courses.lumenlearning.com
Electrons Biology for Majors I Valence Electrons Does Chlorine Ion One with two 1s electrons, one with two 2s electrons and six 2p electrons,. (in table salt, this electron comes from the sodium atom.) e− + cl. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. There are one chlorine atom and three. Therefore, the electron configuration of chlorine will be. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. There are one chlorine atom and three. The electron configuration. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does BrF5 Have? Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. Only one more electron is needed to achieve an octet in chlorine’s valence shell. There are one chlorine atom and three. Chlorine has 7. Valence Electrons Does Chlorine Ion.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Valence Electrons Does Chlorine Ion Chlorine has 7 valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5.. Valence Electrons Does Chlorine Ion.
From chemtech-us.com
15 Interesting Facts About Chlorine Valence Electrons Does Chlorine Ion (in table salt, this electron comes from the sodium atom.) e− + cl. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. The. Valence Electrons Does Chlorine Ion.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Valence Electrons Does Chlorine Ion As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine has 7 valence electrons. (in table salt, this electron comes from the sodium atom.) e− + cl. Therefore, the electron configuration of chlorine. Valence Electrons Does Chlorine Ion.
From byjus.com
In the electron dot structure, the valence shell electrons are Valence Electrons Does Chlorine Ion There are one chlorine atom and three. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. Chlorine has 7 valence electrons. (in table salt, this electron comes from the sodium atom.) e− + cl. As another example,. Valence Electrons Does Chlorine Ion.
From valenceelectrons.com
How Many Valence Electrons Does PCl5 Have? Valence Electrons Does Chlorine Ion 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. Total number of electrons of the valance shells. Valence Electrons Does Chlorine Ion.
From favpng.com
Lewis Structure Chlorine Valence Electron Diagram, PNG, 2000x2000px Valence Electrons Does Chlorine Ion There are one chlorine atom and three. Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion. The electron configuration of chlorine refers to the arrangement of electrons in the chlorine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence Electrons Does Chlorine Ion.