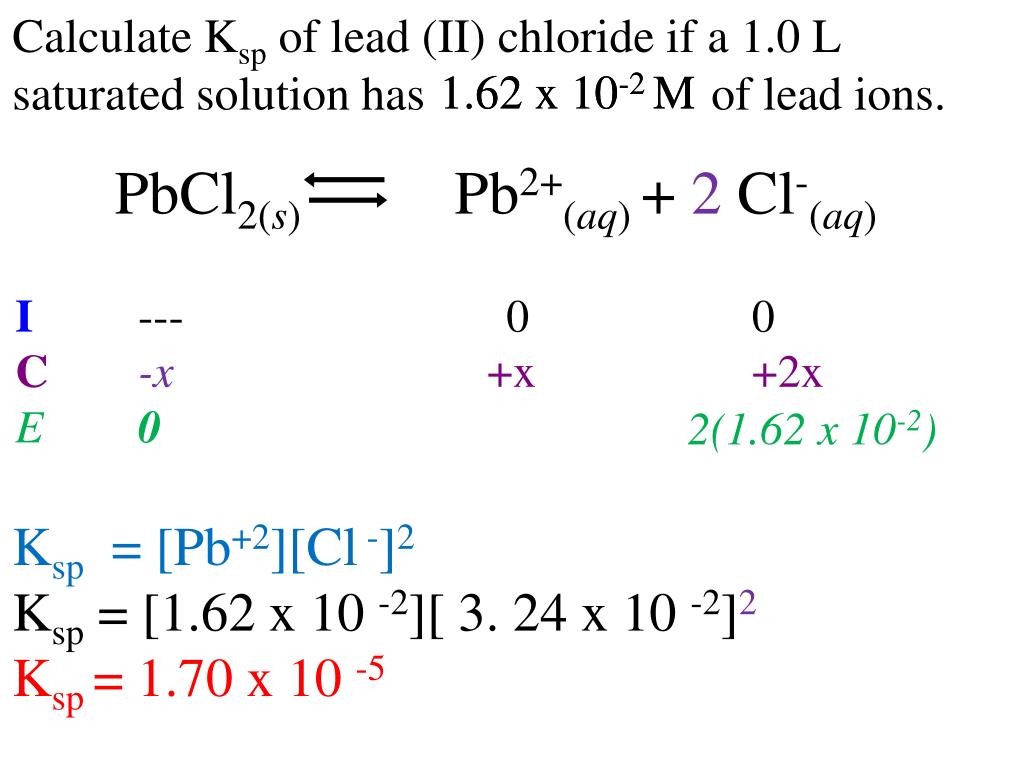
Lead Chloride Product Constant . What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Find out how to calculate ksp from. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c.
from www.slideserve.com
Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Find out how to calculate ksp from. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution.
PPT Solubility Equilibria PowerPoint Presentation, free download ID4857786
Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Find out how to calculate ksp from. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general.
From www.slideserve.com
PPT The Solubility Product Constant, K sp PowerPoint Presentation, free download ID161550 Lead Chloride Product Constant What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for various. Lead Chloride Product Constant.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ).. Lead Chloride Product Constant.
From www.coursehero.com
[Solved] Calculate the solubility product constant for lead(II) chloride, if... Course Hero Lead Chloride Product Constant Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out. Lead Chloride Product Constant.
From www.slideserve.com
PPT Solubility Product Constants PowerPoint Presentation, free download ID205777 Lead Chloride Product Constant Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out how to calculate ksp from. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at. Lead Chloride Product Constant.
From www.wikidoc.org
Lead wikidoc Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out how to calculate ksp from. Learn how the solubility of lead chloride (pbcl₂) is influenced. Lead Chloride Product Constant.
From www.coursehero.com
[Solved] Solubility Product Constants 25.C Name Formula KSp Name Formula barium carbonate BaCO3 Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Find out. Lead Chloride Product Constant.
From www.chegg.com
The Solubility Product of Lead(II) Chloride The Lead Chloride Product Constant What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general.. Lead Chloride Product Constant.
From www.toppr.com
Determine the solubilities of silver chromate, barium chromate, ferric hydroxide, lead chloride Lead Chloride Product Constant What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Find out how to calculate ksp from. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25. Lead Chloride Product Constant.
From testbook.com
Lead (II) Chloride Formula Structure, Properties, Uses & More Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Learn how to use. Lead Chloride Product Constant.
From www.numerade.com
SOLVED TABLE 17.2 Selected SolubilityProduct Constants (Ksp) at 25°C Compound Formula Ksp Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25. Lead Chloride Product Constant.
From www.chegg.com
Solved TABLE 17.2 Selected Solubility Product Constants Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out how to calculate ksp from. What is the lead concentration for a saturated solution of lead(ii)bromide, and then. Lead Chloride Product Constant.
From www.numerade.com
SOLVED Given the following salts of lead(I) and their solubility product constants, which would Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Find out how to calculate ksp from. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2),. Lead Chloride Product Constant.
From chemicaldb.netlify.app
Lead hydroxide chloride solubility Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. Find out how to calculate. Lead Chloride Product Constant.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID4857786 Lead Chloride Product Constant Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. Find out how to calculate ksp from. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and. Lead Chloride Product Constant.
From www.gkseries.com
Lead chloride is Lead Chloride Product Constant Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration. Lead Chloride Product Constant.
From www.chegg.com
Solved The Solubility Product Constant for lead chloride is Lead Chloride Product Constant Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. Find out how to calculate ksp from. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions. Lead Chloride Product Constant.
From www.chegg.com
Solved 7. The solubility product constant for lead chloride Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's.. Lead Chloride Product Constant.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is. Lead Chloride Product Constant.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID4347161 Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how the. Lead Chloride Product Constant.
From www.researchgate.net
Solubility products of selected lead minerals. Download Table Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility. Lead Chloride Product Constant.
From www.coursehero.com
[Solved] Solubility Product Constants 25.C Name Formula KSp Name Formula barium carbonate BaCO3 Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how the. Lead Chloride Product Constant.
From wizedu.com
Determine the molar solubility of lead (II) chloride in each of the following WizEdu Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in. Lead Chloride Product Constant.
From collegedunia.com
Lead II Chloride Formula Properties, Structure, Examples Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2. Lead Chloride Product Constant.
From www.chegg.com
Solved The Solubility Product Constant for lead chloride is Lead Chloride Product Constant Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. Find out how to calculate ksp from. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le. Lead Chloride Product Constant.
From www.wikidoc.org
Lead wikidoc Lead Chloride Product Constant What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out. Lead Chloride Product Constant.
From studylib.net
Solubility Product Constant of Lead(II)chloride C12413 Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Find out how to calculate ksp from. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including. Lead Chloride Product Constant.
From www.toppr.com
Lead II Chloride Formula Definition, Concepts and Examples Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Find out how to calculate ksp from. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Learn how the solubility of lead chloride (pbcl₂) is influenced. Lead Chloride Product Constant.
From www.fishersci.com
Lead Chloride, Powder, 97, Spectrum Chemical, Quantity 500 g Fisher Scientific Lead Chloride Product Constant 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Find out how. Lead Chloride Product Constant.
From www.youtube.com
How to write Molecular formula of lead chloride Chemical formula of Lead chloride YouTube Lead Chloride Product Constant What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). Find out how to calculate ksp from.. Lead Chloride Product Constant.
From www.slideserve.com
PPT Solubility Product Constant PowerPoint Presentation, free download ID2438580 Lead Chloride Product Constant Find out how to calculate ksp from. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c in appendix b of general. Learn how to use solubility product constants (ksp) to describe saturated solutions. Lead Chloride Product Constant.
From encyclopedia.pub
Compounds of Lead Encyclopedia MDPI Lead Chloride Product Constant Find out how to calculate ksp from. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ).. Lead Chloride Product Constant.
From www.numerade.com
SOLVEDDetermine the solubilities of silver chromate, barium chromate, ferric hydroxide, lead Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. Find out how to calculate ksp from. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically. Lead Chloride Product Constant.
From www.slideserve.com
PPT PRECIPITATION REACTIONS Solubility of Salts Section 18.4 PowerPoint Presentation ID4565037 Lead Chloride Product Constant Learn how to use solubility product constants (ksp) to describe saturated solutions of ionic compounds. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. What is the lead concentration for. Lead Chloride Product Constant.
From www.glentham.com
Lead(II) chloride, 99 (CAS 7758954) Glentham Life Sciences Lead Chloride Product Constant Find out how to calculate ksp from. Learn how the solubility of lead chloride (pbcl₂) is influenced by temperature, ph, and other ions in the solution. What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 133 rows find the solubility product constant (ksp) for cadmium fluoride (cdf2) and other compounds at 25°c. Lead Chloride Product Constant.
From fphoto.photoshelter.com
precipitation lead chloride chemistry solubility Fundamental Photographs The Art of Science Lead Chloride Product Constant 176 rows find the solubility product constant (ksp) of various compounds, including manganese iodide (mni2), at 25 °c. 133 rows find the solubility product constant (ksp) for various compounds in water at 25°c, including barium chloride (bacl 2 ). What is the lead concentration for a saturated solution of lead(ii)bromide, and then mathematically demonstrate le chatlier's. 133 rows find the. Lead Chloride Product Constant.