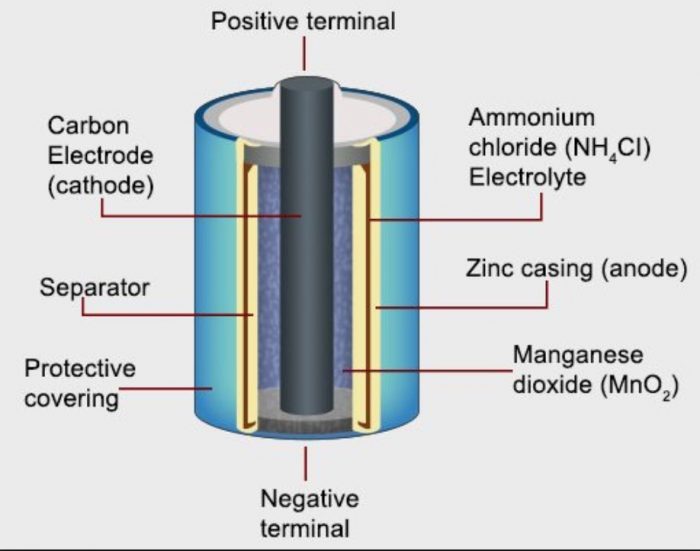
How Does A Battery Electrolyte Work . The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. To accept and release energy, a battery is coupled to an external circuit. How does a battery work? Two terminals made of different chemicals (typically metals), the anode and the cathode; It is a solution or substance that enables the flow of. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. Slowly, the chemicals inside it are. Electrons move through the circuit, while simultaneously ions (atoms or. A battery electrolyte is a crucial component in the functioning of batteries. Electrolyte also comes in a polymer, as used in. There are three main components of a battery: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Whether it is a liquid, gel, or.
from classnotes.org.in
The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. Two terminals made of different chemicals (typically metals), the anode and the cathode; The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. There are three main components of a battery: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Slowly, the chemicals inside it are. To accept and release energy, a battery is coupled to an external circuit. How does a battery work? It is a solution or substance that enables the flow of. Electrolyte also comes in a polymer, as used in.
Batteries Chemistry, Class 12, Electro Chemistry
How Does A Battery Electrolyte Work Slowly, the chemicals inside it are. Slowly, the chemicals inside it are. Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrons move through the circuit, while simultaneously ions (atoms or. To accept and release energy, a battery is coupled to an external circuit. Electrolyte also comes in a polymer, as used in. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. Whether it is a liquid, gel, or. It is a solution or substance that enables the flow of. There are three main components of a battery: How does a battery work? A battery electrolyte is a crucial component in the functioning of batteries. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety.
From www.slideserve.com
PPT The Lead Acid Electric Battery PowerPoint Presentation, free How Does A Battery Electrolyte Work To accept and release energy, a battery is coupled to an external circuit. A battery electrolyte is a crucial component in the functioning of batteries. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. There are three main components of a battery: It is a solution or substance that enables the flow of.. How Does A Battery Electrolyte Work.
From hyetlithium.com
Solidstate Liion batteries HyET Lithium How Does A Battery Electrolyte Work It is a solution or substance that enables the flow of. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Two terminals made of different chemicals (typically metals), the anode and the cathode; The electrolyte is a vital component that directly influences a battery’s. How Does A Battery Electrolyte Work.
From skill-lync.com
Understanding Different Battery Chemistry SkillLync How Does A Battery Electrolyte Work When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrolyte also comes in a polymer,. How Does A Battery Electrolyte Work.
From www.huntkeyenergystorage.com
Battery electrolyte an important component of the battery Huntkey How Does A Battery Electrolyte Work The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. To accept and release energy, a battery is coupled to an external circuit. Electrons move through. How Does A Battery Electrolyte Work.
From www.chemistryworld.com
Solid electrolyte boosts liquid metal battery Research Chemistry World How Does A Battery Electrolyte Work Two terminals made of different chemicals (typically metals), the anode and the cathode; The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Slowly, the chemicals. How Does A Battery Electrolyte Work.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry How Does A Battery Electrolyte Work When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Electrons move through the circuit, while simultaneously ions (atoms or. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. The electrolyte is a vital component that directly influences. How Does A Battery Electrolyte Work.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts How Does A Battery Electrolyte Work There are three main components of a battery: The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. Slowly, the chemicals inside it are. Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrolyte also comes in a polymer, as used in. Whether it is a liquid,. How Does A Battery Electrolyte Work.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode How Does A Battery Electrolyte Work Slowly, the chemicals inside it are. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. There are three main components of a battery: How does a battery work? Whether it is a liquid, gel, or. The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry. How Does A Battery Electrolyte Work.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace How Does A Battery Electrolyte Work To accept and release energy, a battery is coupled to an external circuit. There are three main components of a battery: Slowly, the chemicals inside it are. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. When you connect a battery's two electrodes into a circuit (for example, when you put one in. How Does A Battery Electrolyte Work.
From www.dnkpower.com
Basics on Lithium Battery Electrolyte Lithium ion Battery How Does A Battery Electrolyte Work A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. How does a battery work? The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. Electrolyte also comes in a polymer, as used in. Electrons move through the circuit, while simultaneously ions (atoms or. It. How Does A Battery Electrolyte Work.
From www.uetechnologies.com
What Is The Electrolyte In A Battery? (Working Principle & Battery Types) How Does A Battery Electrolyte Work Two terminals made of different chemicals (typically metals), the anode and the cathode; A battery electrolyte is a crucial component in the functioning of batteries. Slowly, the chemicals inside it are. It is a solution or substance that enables the flow of. How does a battery work? Whether it is a liquid, gel, or. There are three main components of. How Does A Battery Electrolyte Work.
From article.murata.com
Part 4 What are solidstate batteries? An expert explains the basics How Does A Battery Electrolyte Work There are three main components of a battery: A battery electrolyte is a crucial component in the functioning of batteries. The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte. How Does A Battery Electrolyte Work.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID6585303 How Does A Battery Electrolyte Work The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the cathode; A battery electrolyte is a crucial component in the functioning of batteries. Electrons move through the circuit, while simultaneously ions (atoms or. The electrolyte. How Does A Battery Electrolyte Work.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors How Does A Battery Electrolyte Work To accept and release energy, a battery is coupled to an external circuit. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. Electrolyte also comes in a polymer, as used in. The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. How does a battery. How Does A Battery Electrolyte Work.
From www.youtube.com
Tutorial 15Battery with ionic liquidbased electrolyte YouTube How Does A Battery Electrolyte Work A battery electrolyte is a crucial component in the functioning of batteries. Slowly, the chemicals inside it are. There are three main components of a battery: Electrons move through the circuit, while simultaneously ions (atoms or. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. How does a battery work? Two terminals made of. How Does A Battery Electrolyte Work.
From www.mdpi.com
Membranes Free FullText Polymer Electrolytes for Lithium/Sulfur How Does A Battery Electrolyte Work It is a solution or substance that enables the flow of. Slowly, the chemicals inside it are. There are three main components of a battery: When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. The electrolyte of a battery consists of soluble salts, acids. How Does A Battery Electrolyte Work.
From www.takomabattery.com
What is an electrolyte a component of battery The Best lithium ion How Does A Battery Electrolyte Work It is a solution or substance that enables the flow of. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. Slowly, the chemicals inside it are. A battery electrolyte is a crucial component in the functioning of batteries. There are three main components of a battery: When you connect a battery's two electrodes into. How Does A Battery Electrolyte Work.
From large.stanford.edu
Solid Electrolyte Interface (SEI) in Sodium Ion Batteries How Does A Battery Electrolyte Work To accept and release energy, a battery is coupled to an external circuit. How does a battery work? When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. The electrolyte. How Does A Battery Electrolyte Work.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo How Does A Battery Electrolyte Work The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. To accept and release energy, a battery is coupled to an external circuit. A battery electrolyte is a crucial component in the functioning of batteries. Slowly, the chemicals inside it are. Two terminals made of different chemicals (typically metals), the anode and the cathode; When. How Does A Battery Electrolyte Work.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in How Does A Battery Electrolyte Work To accept and release energy, a battery is coupled to an external circuit. How does a battery work? Two terminals made of different chemicals (typically metals), the anode and the cathode; Whether it is a liquid, gel, or. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. The electrolyte of a battery consists. How Does A Battery Electrolyte Work.
From www.huntkeyenergystorage.com
Battery electrolyte an important component of the battery Huntkey How Does A Battery Electrolyte Work Electrolyte also comes in a polymer, as used in. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the cathode; The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. To accept and release energy, a battery is coupled to an external circuit. It is. How Does A Battery Electrolyte Work.
From www.takomabattery.com
What is an electrolyte a component of battery The Best lithium ion How Does A Battery Electrolyte Work It is a solution or substance that enables the flow of. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. There are three main components of a battery: To accept and release energy, a battery is coupled to an external circuit. Slowly, the chemicals inside it are. Whether it is a liquid, gel, or.. How Does A Battery Electrolyte Work.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips How Does A Battery Electrolyte Work Electrolyte also comes in a polymer, as used in. Two terminals made of different chemicals (typically metals), the anode and the cathode; It is a solution or substance that enables the flow of. Electrons move through the circuit, while simultaneously ions (atoms or. Whether it is a liquid, gel, or. How does a battery work? The electrolyte is a vital. How Does A Battery Electrolyte Work.
From dragonflyenergy.com
What Is a Battery Electrolyte and How Does It Work? Dragonfly Energy How Does A Battery Electrolyte Work How does a battery work? The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. A battery electrolyte is a crucial component in the functioning of batteries. Electrolyte also comes in a polymer, as used in. It is a solution or substance that enables the flow of. The electrolyte is a. How Does A Battery Electrolyte Work.
From www.targray.com
Battery Electrolyte (LiPF6) for Liion Manufacturers Targray How Does A Battery Electrolyte Work Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrolyte also comes in a polymer, as used in. There are three main components of a battery: To accept and release energy, a battery is coupled to an external circuit. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. It. How Does A Battery Electrolyte Work.
From savvybatteries.com
What Is Battery Electrolyte And How Does It Work? How Does A Battery Electrolyte Work It is a solution or substance that enables the flow of. A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. To accept and release energy, a battery is coupled to an external circuit. Electrolyte also comes in a polymer, as used in. Two terminals made of different chemicals (typically metals), the anode and. How Does A Battery Electrolyte Work.
From powerclues.com
What is the Purpose of Battery Electrolytes? (How Do Battery How Does A Battery Electrolyte Work Electrolyte also comes in a polymer, as used in. How does a battery work? The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. Slowly, the chemicals inside it are. Two terminals made of different chemicals (typically metals), the anode and the cathode; There are three main components of a battery:. How Does A Battery Electrolyte Work.
From slideplayer.com
PHYS 1444 Lecture 6 Thursday June 21, 2012 Dr. Andrew Brandt ppt How Does A Battery Electrolyte Work Whether it is a liquid, gel, or. Electrolyte also comes in a polymer, as used in. Two terminals made of different chemicals (typically metals), the anode and the cathode; To accept and release energy, a battery is coupled to an external circuit. How does a battery work? A battery electrolyte is a crucial component in the functioning of batteries. The. How Does A Battery Electrolyte Work.
From ul.org
What Are LithiumIon Batteries? UL Research Institutes How Does A Battery Electrolyte Work Slowly, the chemicals inside it are. A battery electrolyte is a crucial component in the functioning of batteries. Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrons move through the circuit, while simultaneously ions (atoms or. Electrolyte also comes in a polymer, as used in. To accept and release energy, a battery is coupled to. How Does A Battery Electrolyte Work.
From www.electricity-magnetism.org
Composition of Leadacid Battery Anode, Cathode & Electrolyte How Does A Battery Electrolyte Work The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. A battery electrolyte is a crucial component in the functioning of batteries. Electrons move through the circuit, while simultaneously ions (atoms or. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the cathode; It is. How Does A Battery Electrolyte Work.
From www.pv-magazine.com
New polymer electrolyte for lithiummetal batteries pv magazine How Does A Battery Electrolyte Work Electrons move through the circuit, while simultaneously ions (atoms or. Whether it is a liquid, gel, or. Two terminals made of different chemicals (typically metals), the anode and the cathode; The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety. A battery electrolyte is a crucial component in the functioning of batteries. The electrolyte of. How Does A Battery Electrolyte Work.
From www.eqmagpro.com
How do lithiumion batteries work? The Leading Solar Magazine In India How Does A Battery Electrolyte Work A battery has three major components —the positive terminal (cathode), the negative terminal (and)e, and an. When you connect a battery's two electrodes into a circuit (for example, when you put one in a flashlight), the electrolyte starts buzzing with activity. Slowly, the chemicals inside it are. It is a solution or substance that enables the flow of. A battery. How Does A Battery Electrolyte Work.
From dragonflyenergy.com
What Is Battery Electrolyte and How Does It Work? Dragonfly Energy How Does A Battery Electrolyte Work Slowly, the chemicals inside it are. To accept and release energy, a battery is coupled to an external circuit. There are three main components of a battery: Whether it is a liquid, gel, or. Electrolyte also comes in a polymer, as used in. A battery electrolyte is a crucial component in the functioning of batteries. It is a solution or. How Does A Battery Electrolyte Work.
From dragonflyenergy.com
What Is a Battery Electrolyte and How Does It Work? Dragonfly Energy How Does A Battery Electrolyte Work The electrolyte of a battery consists of soluble salts, acids or other bases in liquid, gelled and dry formats. It is a solution or substance that enables the flow of. Slowly, the chemicals inside it are. Electrolyte also comes in a polymer, as used in. The electrolyte is a vital component that directly influences a battery’s performance, efficiency, and safety.. How Does A Battery Electrolyte Work.
From www.researchgate.net
The principle of the lithiumion battery (LiB) showing the How Does A Battery Electrolyte Work Electrolyte also comes in a polymer, as used in. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the cathode; Electrons move through the circuit, while simultaneously ions (atoms or. Whether it is a liquid, gel, or. A battery electrolyte is a crucial component in the functioning of batteries. The. How Does A Battery Electrolyte Work.