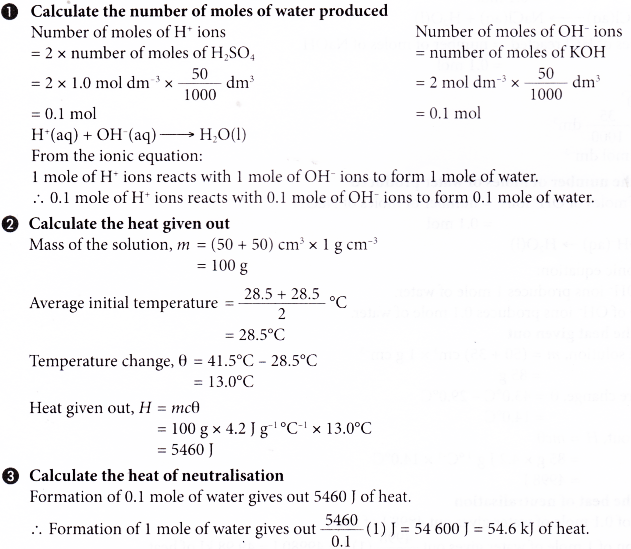
Lab Report Heat Of Neutralization . During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. A suitable reaction for this. These heat (energy) changes are related to the enthalpy. This quantity of heat is. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. When a reaction consumes heat, the temperature inside the calorimeter will decrease. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. Heat of neutralization lab report. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water.
from mehndidesign.zohal.cc
These heat (energy) changes are related to the enthalpy. Heat of neutralization lab report. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. A suitable reaction for this. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. When a reaction consumes heat, the temperature inside the calorimeter will decrease. This quantity of heat is.
Enthalpy Of Neutralization Lab Report Docx The City University ZOHAL
Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. Heat of neutralization lab report. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. When a reaction consumes heat, the temperature inside the calorimeter will decrease. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. These heat (energy) changes are related to the enthalpy. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. This quantity of heat is. A suitable reaction for this. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water.
From www.studocu.com
Enthalpy of Neutralization Lab Report Name Anika Anjum Partner Lab Report Heat Of Neutralization Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. During this lab you will first access the google sheet (link. Lab Report Heat Of Neutralization.
From www.studocu.com
PreLab report heat of neutralization Name Noemi Camacho Lab 1 In Lab Report Heat Of Neutralization The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. These heat (energy) changes are related to the enthalpy. This quantity of heat is. Objectives •. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Lab Report Heat Of Neutralization Heat of neutralization lab report. This quantity of heat is. When a reaction consumes heat, the temperature inside the calorimeter will decrease. A suitable reaction for this. These heat (energy) changes are related to the enthalpy. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. In this experiment,. Lab Report Heat Of Neutralization.
From studylib.net
Heat of Neutralization Lab Lab Report Heat Of Neutralization The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. A suitable reaction for this. During this lab you will first access the google sheet. Lab Report Heat Of Neutralization.
From oneclass.com
OneClass Heat of Neutralization Lab Partner Date Reaction 1 Reaction 2 Lab Report Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. Heat of neutralization lab report. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen. Lab Report Heat Of Neutralization.
From mehndidesign.zohal.cc
Enthalpy Of Neutralization Lab Report Docx The City University ZOHAL Lab Report Heat Of Neutralization Heat of neutralization lab report. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. These heat (energy) changes are related to the enthalpy. This quantity of heat is. When a reaction consumes heat, the temperature inside the calorimeter will decrease. During this lab you will first access. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION CHEM 3H1 USTP Heat of Neutralization Lab Report Studypool Lab Report Heat Of Neutralization The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. In this experiment, you will measure the heat of neutralization when. Lab Report Heat Of Neutralization.
From www.studocu.com
Lab Report Heat of Neutralization.docx StuDocu Lab Report Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. When a reaction consumes heat, the temperature inside the calorimeter will decrease. This quantity of heat is.. Lab Report Heat Of Neutralization.
From www.academia.edu
(PDF) HEAT OF NEUTRALIZATION LAB REPORT afifah fauzi Academia.edu Lab Report Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. These heat (energy) changes are related to the enthalpy. When a reaction consumes heat, the temperature. Lab Report Heat Of Neutralization.
From www.bartleby.com
Answered This the table data of Neutralization… bartleby Lab Report Heat Of Neutralization During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. This quantity of heat is. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. A suitable reaction for this. When a reaction consumes heat, the. Lab Report Heat Of Neutralization.
From www.chegg.com
REPORT SHEET EXPERIMENT 28 Heat of Neutralization °C Lab Report Heat Of Neutralization These heat (energy) changes are related to the enthalpy. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Heat of. Lab Report Heat Of Neutralization.
From www.scribd.com
Physical Chemistry Laboratory Report Experiment 3 Heat of Lab Report Heat Of Neutralization Heat of neutralization lab report. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. This quantity of heat is. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. In this. Lab Report Heat Of Neutralization.
From www.slideshare.net
Heat of Neutralization Lab Report PDF Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. This quantity of heat is. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Lab Report Heat Of Neutralization Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. This quantity of heat is. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. Design a preliminary procedure to determine the. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Heat of Neutralization A. Lab Report Heat Of Neutralization Heat of neutralization lab report. This quantity of heat is. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. A suitable reaction for this. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and. Lab Report Heat Of Neutralization.
From studylib.net
Determining the Enthalpy of a Neutralization Reaction Work Sheet Lab Report Heat Of Neutralization A suitable reaction for this. This quantity of heat is. When a reaction consumes heat, the temperature inside the calorimeter will decrease. These heat (energy) changes are related to the enthalpy. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. Heat of neutralization lab report. The. Lab Report Heat Of Neutralization.
From www.scribd.com
Heat of Neutralization Lab Report PDF Sodium Hydroxide Mole (Unit) Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. A suitable reaction for this. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Lab Report Heat Of Neutralization Heat of neutralization lab report. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. These heat (energy) changes are related to the enthalpy. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react. Lab Report Heat Of Neutralization.
From www.studocu.com
Lab 6 report lab Thermochemistry Heat of Neutralization and Hess’s Lab Report Heat Of Neutralization These heat (energy) changes are related to the enthalpy. Heat of neutralization lab report. When a reaction consumes heat, the temperature inside the calorimeter will decrease. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. The standard enthalpy change of neutralization[9] is the enthalpy change when. Lab Report Heat Of Neutralization.
From www.studocu.com
Thermo Lab 2 Lab Report Heat of Neutralization Introduction The Lab Report Heat Of Neutralization These heat (energy) changes are related to the enthalpy. Heat of neutralization lab report. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. This quantity of. Lab Report Heat Of Neutralization.
From www.studocu.com
CHE142 HEAT OF Neutralization LAB Report 2022 SCHOOL OF CHEMICAL Lab Report Heat Of Neutralization Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. In this experiment, you will measure the heat of neutralization when. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Lab Report Heat Of Neutralization A suitable reaction for this. This quantity of heat is. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Heat of neutralization lab report.. Lab Report Heat Of Neutralization.
From studylib.net
Neutralization Lab Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. These heat (energy) changes are related to the enthalpy. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an. Lab Report Heat Of Neutralization.
From www.studocu.com
Chem 104 lab 4 report jody REPORT THERMOCHEMISTRY HEAT OF Lab Report Heat Of Neutralization The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. A suitable reaction for this. This quantity of heat is. When a reaction consumes heat, the temperature inside the calorimeter will decrease. Heat of neutralization lab report. These heat (energy) changes are related to the enthalpy. During this. Lab Report Heat Of Neutralization.
From cbselibrary.com
how to calculate heat of neutralization of hcl and naoh Archives CBSE Lab Report Heat Of Neutralization Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. A suitable reaction for this. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. During this lab you will first access the google sheet. Lab Report Heat Of Neutralization.
From www.scribd.com
Lab Heat of Neutralization PDF Lab Report Heat Of Neutralization Heat of neutralization lab report. A suitable reaction for this. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. When a reaction consumes heat, the. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Lab Report Heat Of Neutralization This quantity of heat is. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. A suitable reaction for this. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. During this lab you will. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION Che 142 heat of neutralization lab report Studypool Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. Heat of neutralization lab report.. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved LAB REPORT Name Heat of Neutralization Show your Lab Report Heat Of Neutralization A suitable reaction for this. Heat of neutralization lab report. These heat (energy) changes are related to the enthalpy. In this experiment, you will measure the heat of neutralization when an acid and base react to form 1 mole of water. During this lab you will first access the google sheet (link is in your lab report), the ta will. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved REPORT SHEET Heat of Neutralization 21.4 A. Heat Lab Report Heat Of Neutralization These heat (energy) changes are related to the enthalpy. When a reaction consumes heat, the temperature inside the calorimeter will decrease. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. This quantity of heat is. A suitable reaction for this. In this. Lab Report Heat Of Neutralization.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. This quantity of heat is. During this lab you will first access the google sheet (link is in your lab report), the ta will perform the. The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard.. Lab Report Heat Of Neutralization.
From www.studocu.com
Lab 4 heat of neutralization Chemical Engineering TECHNICAL REPORT Lab Report Heat Of Neutralization Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. When a reaction consumes heat, the temperature inside the calorimeter. Lab Report Heat Of Neutralization.
From www.scribd.com
Lab 10_Heat of Reaction for the Neutralization of Hydrochloric Acid Lab Report Heat Of Neutralization The standard enthalpy change of neutralization[9] is the enthalpy change when solutions of an acid and an alkali react together under standard. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. This quantity of heat is. When a reaction consumes heat, the temperature inside the calorimeter. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION CHEM 3H1 USTP Heat of Neutralization Lab Report Studypool Lab Report Heat Of Neutralization This quantity of heat is. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a styrofoam cup calorimeter using the heat of. Heat of neutralization lab report. A suitable reaction for this. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and. Lab Report Heat Of Neutralization.
From www.studypool.com
SOLUTION C Lab 10 Thermodynamics Heat Of Neutralization Studypool Lab Report Heat Of Neutralization When a reaction consumes heat, the temperature inside the calorimeter will decrease. Design a preliminary procedure to determine the heat of neutralization for the reaction between 3.0 m hcl and 3.0 m naoh in kj/mol. A suitable reaction for this. Objectives • measure the enthalpy of reaction for the decomposition of hydrogen peroxide • measure the heat capacity of a. Lab Report Heat Of Neutralization.