Why Is Dry Ice So Important . through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. dry ice is the solid form of carbon dioxide (co₂). unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. This makes it useful for a wide range of applications, such as freezing and preserving food or other. Instead, at room temperature, it changes.
from www.howitworksdaily.com
through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. This makes it useful for a wide range of applications, such as freezing and preserving food or other. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice is the solid form of carbon dioxide (co₂). Instead, at room temperature, it changes. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream.
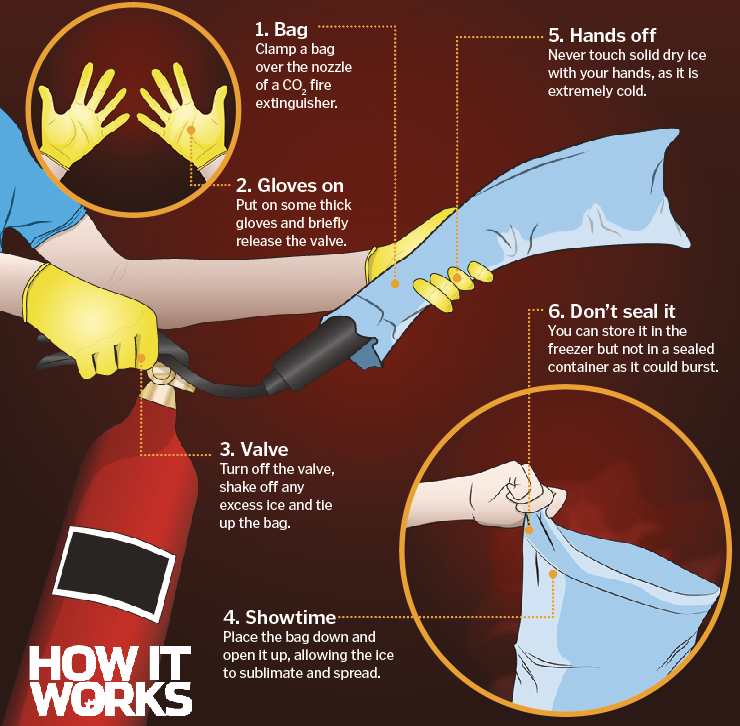
What is dry ice? How It Works
Why Is Dry Ice So Important Instead, at room temperature, it changes. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. This makes it useful for a wide range of applications, such as freezing and preserving food or other. Instead, at room temperature, it changes. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream.
From huntingwaterfalls.com
How Is Dry Ice Made From Factory To Your Home Why Is Dry Ice So Important This makes it useful for a wide range of applications, such as freezing and preserving food or other. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used. Why Is Dry Ice So Important.
From www.lake-link.com
The Cold Hard Facts About Using Dry Ice In Coolers Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. This makes it useful for a wide range of applications, such as freezing and preserving food or. Why Is Dry Ice So Important.
From www.slideserve.com
PPT Dry Ice PowerPoint Presentation, free download ID6103220 Why Is Dry Ice So Important Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. Instead, at room temperature, it changes. This makes it useful for a wide range of applications, such as freezing and preserving food or other. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. through a process. Why Is Dry Ice So Important.
From tomcosystems.com
types of dry ice infographic Why Is Dry Ice So Important dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. unlike the ice cubes in. Why Is Dry Ice So Important.
From cbnc.com
What is dry ice and why is it suddenly so important? CBNC Why Is Dry Ice So Important dry ice is the solid form of carbon dioxide (co₂). through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. This makes it useful for a wide range of applications, such as freezing. Why Is Dry Ice So Important.
From www.youtube.com
What is the chemical formula of dry ice ? QnA Explained YouTube Why Is Dry Ice So Important Instead, at room temperature, it changes. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. This makes it useful for a wide range of applications, such as freezing and preserving food or other. dry ice is the solid form of carbon dioxide (co₂). dry ice,. Why Is Dry Ice So Important.
From www.howitworksdaily.com
What is dry ice? How It Works Why Is Dry Ice So Important dry ice is the solid form of carbon dioxide (co₂). Instead, at room temperature, it changes. This makes it useful for a wide range of applications, such as freezing and preserving food or other. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Unlike regular ice,. Why Is Dry Ice So Important.
From outforia.com
How Long Does Dry Ice Last? Essential Tips & Tricks! Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Instead, at room temperature, it changes. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely.. Why Is Dry Ice So Important.
From dryicewa.com.au
Dry Ice Safety Handling Instructions Dry Ice WA Why Is Dry Ice So Important Instead, at room temperature, it changes. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. This makes it useful for a wide range of applications, such. Why Is Dry Ice So Important.
From emergencyice.com
Best Industrial Uses for Dry Ice Dry Ice Delivery Emergency Ice Why Is Dry Ice So Important Instead, at room temperature, it changes. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. dry ice is the solid form of carbon dioxide (co₂). This makes it useful for a wide range of applications, such. Why Is Dry Ice So Important.
From www.youtube.com
What is Dry ice? Uses of Dry Ice How it is made?🤓 Explained By Why Is Dry Ice So Important unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice is the solid form of carbon dioxide (co₂). Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. Instead, at room temperature, it changes. This makes it useful for a wide range of applications, such. Why Is Dry Ice So Important.
From www.longbeachice.com
7 Practical and LesserKnown Uses for Dry Ice Long Beach Ice Why Is Dry Ice So Important dry ice is the solid form of carbon dioxide (co₂). unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. Instead, at room temperature, it changes. This makes it useful for a wide range of applications, such as freezing and preserving food or other. Unlike regular ice, which is frozen water,. Why Is Dry Ice So Important.
From dryicy.com
What is dry ice and what are its uses? Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. dry ice is the solid form of carbon dioxide (co₂). This makes it useful for a. Why Is Dry Ice So Important.
From www.dryiceadvice.com
A Comprehensive List Of Uses for Dry Ice Dry Ice Advice Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at. Why Is Dry Ice So Important.
From eurekaoxygencompany.com
Dry Ice and How to Preserve It Eureka Oxygen Why Is Dry Ice So Important This makes it useful for a wide range of applications, such as freezing and preserving food or other. Instead, at room temperature, it changes. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable. Why Is Dry Ice So Important.
From askanydifference.com
Ice vs Dry Ice Difference and Comparison Why Is Dry Ice So Important unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice. Why Is Dry Ice So Important.
From blog.coldjet.com
What is Dry Ice? Why Is Dry Ice So Important This makes it useful for a wide range of applications, such as freezing and preserving food or other. dry ice is the solid form of carbon dioxide (co₂). unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. Instead, at room temperature, it changes. through a process called, “sublimation,” dry. Why Is Dry Ice So Important.
From dryicewa.com.au
What is Dry Ice and How to Use it Dry Ice WA Why Is Dry Ice So Important Instead, at room temperature, it changes. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. This makes it useful for a wide range of applications, such as freezing and preserving food or other. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting). Why Is Dry Ice So Important.
From blog.coldjet.com
How does dry ice blasting work? Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Instead, at room temperature, it changes. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially. Why Is Dry Ice So Important.
From www.dryicecorp.com
How Dry Ice is Made Dry Ice Corp Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f),. Why Is Dry Ice So Important.
From www.youtube.com
Four Uses for Dry Ice YouTube Why Is Dry Ice So Important Instead, at room temperature, it changes. This makes it useful for a wide range of applications, such as freezing and preserving food or other. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that. Why Is Dry Ice So Important.
From emergencyice.com
Dry Ice Handling and Storage Safety Tips Emergency Ice Dry Ice Delivery Why Is Dry Ice So Important Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. Instead, at room temperature, it changes. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting). Why Is Dry Ice So Important.
From thebrilliantkitchen.com
How To Make Dry Ice? Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. This makes it useful for a wide range of applications, such as freezing and preserving food or other. Instead, at room temperature, it changes. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that. Why Is Dry Ice So Important.
From www.youtube.com
Why is dry ice so important to a COVID19 vaccine? YouTube Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in. Why Is Dry Ice So Important.
From www.just-food.com
What Format of Dry Ice Does Your Home Delivery Logistics Need? Just Food Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Instead, at room temperature, it changes. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice is the solid form of carbon dioxide (co₂). This makes it. Why Is Dry Ice So Important.
From www.smorescience.com
What Is Dry Ice Made Of? Smore Science Magazine Why Is Dry Ice So Important dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. Unlike regular ice, which is frozen. Why Is Dry Ice So Important.
From www.slideserve.com
PPT Dry Ice PowerPoint Presentation, free download ID6103220 Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at. Why Is Dry Ice So Important.
From dryice.pk
7 Dry Ice Safety and Handling Tips for a Secure Experience Why Is Dry Ice So Important dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in. Why Is Dry Ice So Important.
From reefer-ex.in
What is Dry Ice Temperature & How it is calculated Dry Ice Formula Why Is Dry Ice So Important Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. Instead, at room temperature, it changes. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. This makes it useful for a wide range of applications, such as freezing and preserving food or other.. Why Is Dry Ice So Important.
From www.thoughtco.com
Dry Ice Composition and Uses Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour. Why Is Dry Ice So Important.
From houzzmedia.com
What Is Dry Ice Used For? All You need to Know About Dry Ice Why Is Dry Ice So Important This makes it useful for a wide range of applications, such as freezing and preserving food or other. dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant,. Why Is Dry Ice So Important.
From kimia.co.uk
Why Is Dry Ice Suddenly So Important Why Is Dry Ice So Important unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Instead, at room temperature, it changes. dry ice, carbon dioxide in its solid form, a dense, snowlike substance that. Why Is Dry Ice So Important.
From cksupply.com
The Many Dry Ice Uses CK Supply Why Is Dry Ice So Important Instead, at room temperature, it changes. dry ice is the solid form of carbon dioxide (co₂). dry ice, carbon dioxide in its solid form, a dense, snowlike substance that sublimes (passes directly into the vapour without melting) at −78.5 °c (−109.3 °f), used as a refrigerant, especially during shipping of perishable products such as meats or ice cream.. Why Is Dry Ice So Important.
From techiescientist.com
17 Uses of Dry Ice Commercial, Industrial, & Scientific Uses Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Instead, at room temperature, it changes. dry ice is the solid form of carbon dioxide (co₂). Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. unlike the ice cubes in a. Why Is Dry Ice So Important.
From www.slideserve.com
PPT Dry Ice PowerPoint Presentation, free download ID6103220 Why Is Dry Ice So Important through a process called, “sublimation,” dry ice changes directly from a solid to a gas in normal atmospheric conditions without going. Unlike regular ice, which is frozen water, dry ice skips the liquid phase entirely. unlike the ice cubes in a cold drink, dry ice doesn't melt to become liquid at all. dry ice, carbon dioxide in. Why Is Dry Ice So Important.