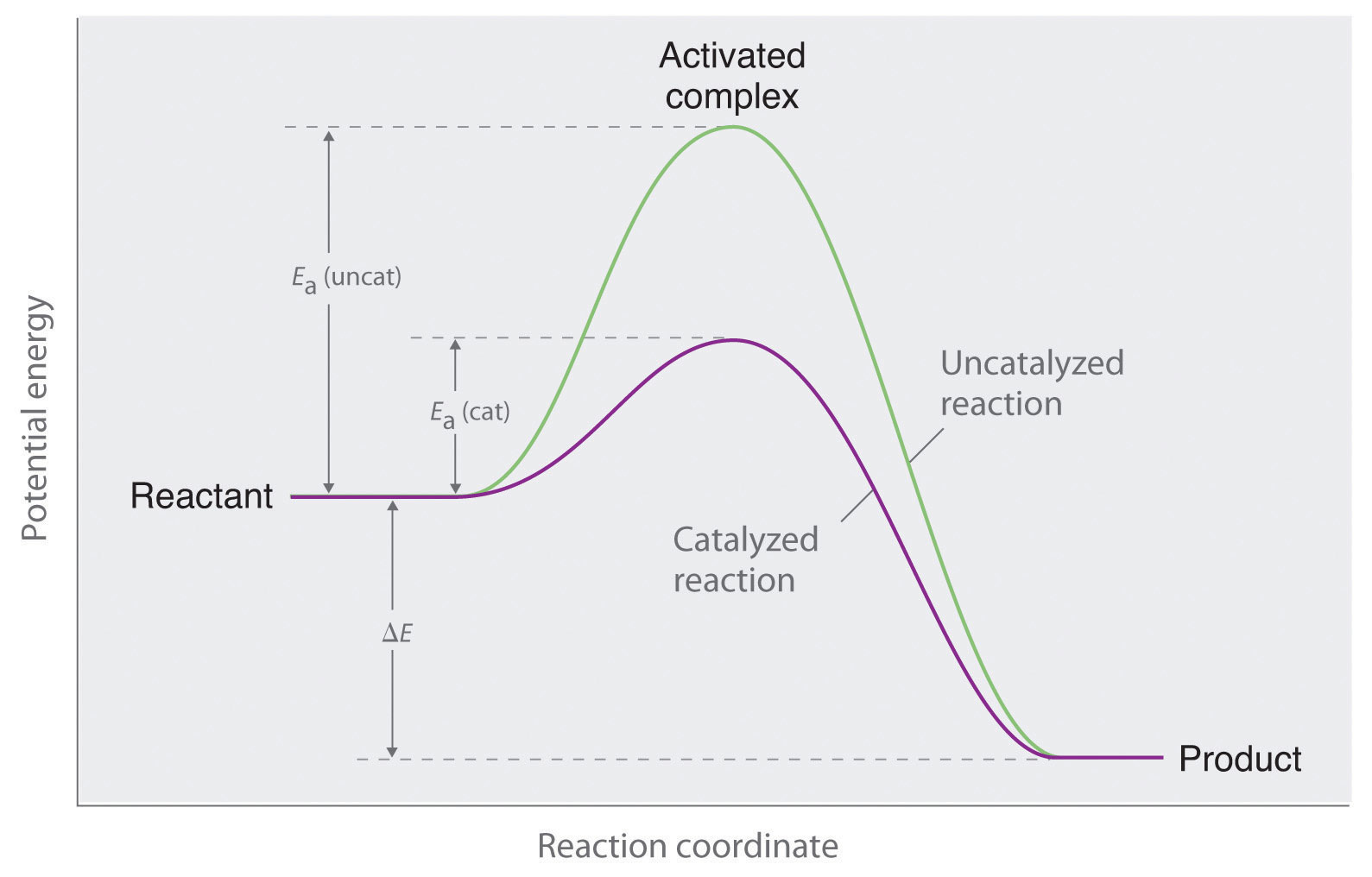
Catalytic Function Meaning . Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. List examples of catalysis in natural. We start the discussion with a definition: A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysis is the process of speeding up a. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A very small amount of catalyst is required. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams.
from 2012books.lardbucket.org
In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysts can be homogenous (in the same phase as the reactants) or. A very small amount of catalyst is required. Catalysis is the process of speeding up a. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that increases the rate at which a chemical reaction approaches. List examples of catalysis in natural.
Catalysis
Catalytic Function Meaning Catalysis is the process of speeding up a. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. We start the discussion with a definition: Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyst is a substance that increases the rate at which a chemical reaction approaches. A very small amount of catalyst is required. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is the process of speeding up a. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts can be homogenous (in the same phase as the reactants) or.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalytic Function Meaning Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process of speeding up a. Catalysts can be homogenous (in the same phase as the reactants) or. A very small. Catalytic Function Meaning.
From golchaminerals.com
Ceramics & Catalytic Converters Golcha Group Catalytic Function Meaning We start the discussion with a definition: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText Recovery/Reuse of Heterogeneous Supported Catalytic Function Meaning List examples of catalysis in natural. A very small amount of catalyst is required. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts can be homogenous (in the same phase as the reactants) or. We start the discussion with a definition: Explain the function of a. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText A General Overview of Support Materials Catalytic Function Meaning Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that increases the rate at which a chemical reaction approaches. We start the discussion with a definition: Catalysis is a process of increasing the rate. Catalytic Function Meaning.
From fyowexdtd.blob.core.windows.net
Catalyst Biology Cell at Michael Holmes blog Catalytic Function Meaning A very small amount of catalyst is required. Catalysis is the process of speeding up a. We start the discussion with a definition: A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysis is a process of increasing the rate of a. Catalytic Function Meaning.
From giorynakh.blob.core.windows.net
Catalytic Converter Meaning Car at Sandra Curry blog Catalytic Function Meaning A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural. Explain the. Catalytic Function Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Function Meaning Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysis is the process of speeding up. Catalytic Function Meaning.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalytic Function Meaning A very small amount of catalyst is required. A catalyst is a substance that increases the rate at which a chemical reaction approaches. We start the discussion with a definition: Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.. Catalytic Function Meaning.
From www.youtube.com
Catalytic Converter Problems & Replacement YouTube Catalytic Function Meaning A very small amount of catalyst is required. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. In chemistry and. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText Switchable StimuliResponsive Catalytic Function Meaning Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A very small amount of catalyst is required. Catalysis is the process of speeding up a. Explain the function. Catalytic Function Meaning.
From exotuoltx.blob.core.windows.net
Catalyst Meaning Science at Karolyn Roque blog Catalytic Function Meaning Catalysis is the process of speeding up a. A very small amount of catalyst is required. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural. Catalysis is a process of increasing the rate. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText Synthesis of Catalytic Precursors Based on Catalytic Function Meaning Catalysts can be homogenous (in the same phase as the reactants) or. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. We start the discussion with a definition: In chemistry and biology, a catalyst is a substance the increases. Catalytic Function Meaning.
From medium.com
Is body fat really our enemy?. You might be surprised to know that fat Catalytic Function Meaning Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it.. Catalytic Function Meaning.
From 2012books.lardbucket.org
Catalysis Catalytic Function Meaning Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. We start the discussion with a definition: A very small amount of catalyst is required. List examples of catalysis in natural. Catalysis is the process of speeding up a. Catalysis. Catalytic Function Meaning.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalytic Function Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst is a substance that increases. Catalytic Function Meaning.
From pressbooks.bccampus.ca
4.6 Catalysis Chemistry for Chemical Engineers Catalytic Function Meaning Catalysis is the process of speeding up a. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams.. Catalytic Function Meaning.
From www.youtube.com
Catalytic converter Meaning YouTube Catalytic Function Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. List examples of catalysis in natural. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy.. Catalytic Function Meaning.
From www.youtube.com
Catalytic activity Meaning YouTube Catalytic Function Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. List examples of catalysis in natural. We start the discussion with a definition: Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a substance that increases the rate at. Catalytic Function Meaning.
From www.biologyonline.com
Ligase Definition and Examples Biology Online Dictionary Catalytic Function Meaning Catalysts can be homogenous (in the same phase as the reactants) or. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the. Catalytic Function Meaning.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalytic Function Meaning We start the discussion with a definition: Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. Catalytic Function Meaning.
From autocatalystmarket.com
ᐈ Car catalyst functions and significance AutoCatalystMarket Experts USA Catalytic Function Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText Heterogeneous Catalyst Deactivation and Catalytic Function Meaning A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts can be homogenous (in the same phase as the reactants) or. A very small amount of catalyst is required. Catalysis is the process of speeding up a. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText FCC Catalyst Accessibility—A Review Catalytic Function Meaning A very small amount of catalyst is required. List examples of catalysis in natural. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance. Catalytic Function Meaning.
From www.carblogindia.com
What Are Catalytic Converters? How Do They Reduce Emissions? » Car Blog Catalytic Function Meaning We start the discussion with a definition: Catalysis is the process of speeding up a. A catalyst is a substance that increases the rate at which a chemical reaction approaches. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. List examples of catalysis in natural. A very. Catalytic Function Meaning.
From www.creative-enzymes.com
Catalytic Characteristics of Enzymes Creative Enzymes Catalytic Function Meaning List examples of catalysis in natural. We start the discussion with a definition: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts can be homogenous (in the same. Catalytic Function Meaning.
From www.mdpi.com
Catalysts Free FullText Preparation and Photocatalytic Activities Catalytic Function Meaning Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. We start the discussion with a definition: A very small amount of catalyst is required. List examples of catalysis in natural. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that. Catalytic Function Meaning.
From www.youtube.com
Catalytic Meaning YouTube Catalytic Function Meaning We start the discussion with a definition: A very small amount of catalyst is required. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts can be homogenous (in the same phase. Catalytic Function Meaning.
From meaningkosh.com
Catalytic Meaning In Hindi MeaningKosh Catalytic Function Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. List examples of catalysis in natural. We start the discussion with a definition: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that. Catalytic Function Meaning.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalytic Function Meaning List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysts can be homogenous (in the same phase as the reactants) or. A very small amount. Catalytic Function Meaning.
From scales.arabpsychology.com
CATALYTIC VARIABLE Definition & Meaning Catalytic Function Meaning A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is a process of increasing the rate of a chemical. Catalytic Function Meaning.
From exonfxjgk.blob.core.windows.net
Catalytic Converter Noise Video at Serena Bailey blog Catalytic Function Meaning Catalysis is the process of speeding up a. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy.. Catalytic Function Meaning.
From www.questionai.com
Objective 17 Describe the Main Function of an Enzyme (catalyst). 35 Catalytic Function Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. We start the discussion with a definition: List examples of catalysis in natural. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process of. Catalytic Function Meaning.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalytic Function Meaning Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysts can be homogenous. Catalytic Function Meaning.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalytic Function Meaning A very small amount of catalyst is required. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a substance that increases the rate at which a chemical reaction approaches. Catalysis is the process of speeding up a. List examples of catalysis in natural. Catalysts can be homogenous (in. Catalytic Function Meaning.
From www.autopadre.com
Catalytic Converter Location And Understanding Its Importance in Your Catalytic Function Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. Explain the function of a catalyst. Catalytic Function Meaning.