What Is Gas Equation Definition . There are various type of. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law is also known as the general gas equation. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect).
from www.priyamstudycentre.com
The ideal gas law is also known as the general gas equation. There are various type of. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature.
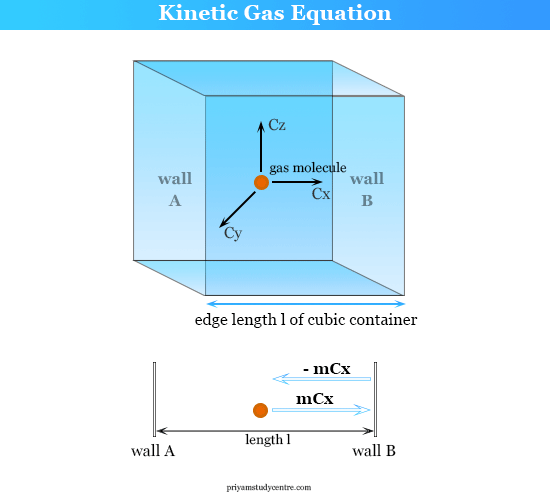
Theory of Gases Gas Equation, Postulates, Formula
What Is Gas Equation Definition In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). The ideal gas law is also known as the general gas equation. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. There are various type of.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. The ideal gas law is also known as the general gas equation.. What Is Gas Equation Definition.
From owlcation.com
The Theories and Behavior of Gas Owlcation What Is Gas Equation Definition The ideal gas law is also known as the general gas equation. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure,. What Is Gas Equation Definition.
From www.youtube.com
Molecular Speed of Gases Formula With Boltzmann's Constant YouTube What Is Gas Equation Definition There are various type of. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law describes the behavior of an. What Is Gas Equation Definition.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Is Gas Equation Definition The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. There are various type of. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In cases where \( \frac{pv}{nrt} \neq 1. What Is Gas Equation Definition.
From www.youtube.com
Derivation of of Gas Equation YouTube What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. The ideal gas law is also known as the general gas equation. It is an equation of state of. What Is Gas Equation Definition.
From www.thoughtco.com
What Is the Ideal Gas Law Definition and Equation? What Is Gas Equation Definition In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. There are various type of. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or. What Is Gas Equation Definition.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit. What Is Gas Equation Definition.
From mavink.com
Formula To Calculate Alveolar Gas Equation What Is Gas Equation Definition The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. There are various type of. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the. What Is Gas Equation Definition.
From www.youtube.com
theory of Gases Assumption Introduction Derivation of What Is Gas Equation Definition In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). The ideal. What Is Gas Equation Definition.
From www.priyamstudycentre.com
Theory of Gases Gas Equation, Postulates, Formula What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all. What Is Gas Equation Definition.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Is Gas Equation Definition There are various type of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. The ideal gas law is also known as the general gas equation. The ideal gas law describes the behavior of an. What Is Gas Equation Definition.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica What Is Gas Equation Definition Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low. What Is Gas Equation Definition.
From www.youtube.com
Theory of gases Lecture 2 Derivation of Gas Equation What Is Gas Equation Definition The ideal gas law is also known as the general gas equation. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. There are various type of. Ideal gas law, relation between the pressure p, volume. What Is Gas Equation Definition.
From www.youtube.com
Gas volumes (AQA GCSE Chemistry) YouTube What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature.. What Is Gas Equation Definition.
From mavink.com
Derive Ideal Gas Equation What Is Gas Equation Definition The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas law, relation between the pressure p, volume v, and temperature t of. What Is Gas Equation Definition.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID What Is Gas Equation Definition The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. The ideal gas law is also known as the general gas equation. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. Ideal gas, a gas that conforms, in physical behavior, to a. What Is Gas Equation Definition.
From www.youtube.com
8.17 How do i derive the theory of gas equation? YouTube What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in. What Is Gas Equation Definition.
From yzymis.blogspot.com
NonIdeal Gases and the Van der Waals Equation What Is Gas Equation Definition There are various type of. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). Ideal gas law, relation between the pressure p, volume v, and temperature t of. What Is Gas Equation Definition.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills What Is Gas Equation Definition Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets. What Is Gas Equation Definition.
From narodnatribuna.info
Example Using Specific Heat To Calculate Ideal Gas What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. There are various type of. It is an equation of state of. What Is Gas Equation Definition.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube What Is Gas Equation Definition In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). There are various type of. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a. What Is Gas Equation Definition.
From www.youtube.com
Lecture 5 Th of gases Collision Frequency and Mean free path What Is Gas Equation Definition The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law. What Is Gas Equation Definition.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy What Is Gas Equation Definition In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. The ideal gas law is also known as the general gas equation. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure,. What Is Gas Equation Definition.
From scoop.eduncle.com
A non ideal gas follows the equation What Is Gas Equation Definition There are various type of. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). The ideal gas law is also known as the general gas equation. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Ideal gas law, relation between the pressure. What Is Gas Equation Definition.
From en.ppt-online.org
The ideal gas equation online presentation What Is Gas Equation Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. In. What Is Gas Equation Definition.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Is Gas Equation Definition In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). There are various type of. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is. What Is Gas Equation Definition.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube What Is Gas Equation Definition There are various type of. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance. What Is Gas Equation Definition.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation What Is Gas Equation Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature. What Is Gas Equation Definition.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is Gas Equation Definition Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law is also known as. What Is Gas Equation Definition.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4144868 What Is Gas Equation Definition In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. The ideal gas law is also known as the general gas equation. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move.. What Is Gas Equation Definition.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download What Is Gas Equation Definition Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is also known as the general. What Is Gas Equation Definition.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii What Is Gas Equation Definition There are various type of. It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. The ideal gas law is also known as the general gas equation. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high. What Is Gas Equation Definition.
From diagramlibraryisadore.z21.web.core.windows.net
Entropy Enthalpy Temperature Equation What Is Gas Equation Definition It is an equation of state of an ideal gas that relates pressure, volume, quantity of gas, and temperature. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect).. What Is Gas Equation Definition.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation What Is Gas Equation Definition In cases where \( \frac{pv}{nrt} \neq 1 \) or if there are multiple sets of. In an ideal gas situation, \( \frac{pv}{nrt} = 1 \) (assuming all gases are ideal or perfect). Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. There are. What Is Gas Equation Definition.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 What Is Gas Equation Definition Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. It is an equation of state of an ideal gas. What Is Gas Equation Definition.