How Chlorine Atom Destroy Ozone . one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. chlorine is able to destroy so much of the ozone because it acts as a catalyst. how ozone is destroyed by cfcs. Ozone can be destroyed more.
from ozotech.com
Ozone can be destroyed more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). chlorine is able to destroy so much of the ozone because it acts as a catalyst. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. how ozone is destroyed by cfcs. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more.
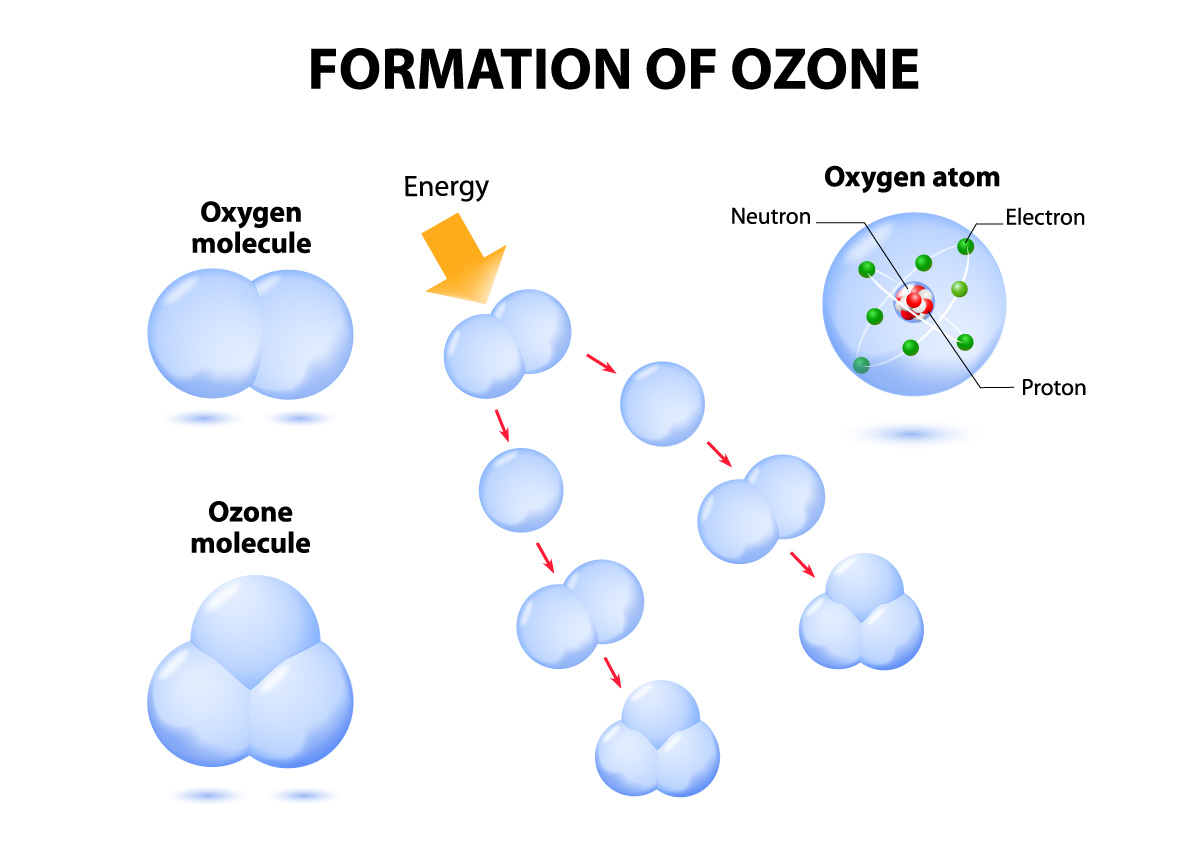
The Ozone Molecule Ozotech, Inc.
How Chlorine Atom Destroy Ozone When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. Ozone can be destroyed more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. chlorine is able to destroy so much of the ozone because it acts as a catalyst. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. how ozone is destroyed by cfcs. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more.
From www.coursehero.com
[Solved] EPA hvac 19. A single chlorine atom can destroy how many ozone... Course Hero How Chlorine Atom Destroy Ozone how ozone is destroyed by cfcs. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. Ozone can be destroyed more. chlorine is able to destroy so much of the ozone because it acts as a catalyst. two scientists,. How Chlorine Atom Destroy Ozone.
From bio1151b.nicerweb.net
cfc.html 54_27ChlorineOzone.jpg How Chlorine Atom Destroy Ozone chlorine is able to destroy so much of the ozone because it acts as a catalyst. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. how ozone is destroyed by cfcs. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. Ozone. How Chlorine Atom Destroy Ozone.
From www.researchgate.net
4. Mechanism of chlorine atom (Cl) release responsible for ozone (O 3... Download Scientific How Chlorine Atom Destroy Ozone Ozone can be destroyed more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. . How Chlorine Atom Destroy Ozone.
From www.worldatlas.com
What Is The Ozone Hole? WorldAtlas How Chlorine Atom Destroy Ozone one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. Ozone can be destroyed more. how ozone. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Objectives Explain how the ozone layer shields Earth from much of the sun’s harmful radiation How Chlorine Atom Destroy Ozone one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. chlorine is able to destroy so much of the ozone because it acts as a catalyst. how ozone is destroyed by cfcs. Ozone can be destroyed more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known. How Chlorine Atom Destroy Ozone.
From esrl.noaa.gov
Scientific Assessment of Ozone Depletion 2006 Twenty Questions and Answers About the Ozone How Chlorine Atom Destroy Ozone how ozone is destroyed by cfcs. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs).. How Chlorine Atom Destroy Ozone.
From www.worldatlas.com
What Is The Ozone Hole? WorldAtlas How Chlorine Atom Destroy Ozone When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. one chlorine atom can destroy over 100,000 ozone molecules. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Ozone. ppt download How Chlorine Atom Destroy Ozone Ozone can be destroyed more. chlorine is able to destroy so much of the ozone because it acts as a catalyst. how ozone is destroyed by cfcs. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known. How Chlorine Atom Destroy Ozone.
From ozotech.com
The Ozone Molecule Ozotech, Inc. How Chlorine Atom Destroy Ozone When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. one atom of chlorine can destroy more than 100,000 ozone molecules, according to. How Chlorine Atom Destroy Ozone.
From slideplayer.com
How to Use This Presentation ppt download How Chlorine Atom Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). how. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Earth’s Atmosphere PowerPoint Presentation, free download ID1398522 How Chlorine Atom Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. how ozone is destroyed by cfcs. . How Chlorine Atom Destroy Ozone.
From www.studocu.com
CHP 9 Class WK 1 1. How many chlorine atoms does it take to destroy 100,000 ozone molecules in How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. Ozone can be destroyed more. chlorine is able to destroy so much of the ozone because it acts as a catalyst. explain. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy stratospheric ozone How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. how ozone is destroyed by cfcs. Ozone can be destroyed more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic”. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT The Ozone Hole PowerPoint Presentation, free download ID524431 How Chlorine Atom Destroy Ozone one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. how ozone is destroyed by cfcs. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. reactive gases containing chlorine. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Ozone. ppt download How Chlorine Atom Destroy Ozone explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. chlorine is able to destroy so much of the ozone because it acts as a catalyst. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. reactive gases containing chlorine and bromine. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Atmosphere and Climate Change ppt download How Chlorine Atom Destroy Ozone Ozone can be destroyed more. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. chlorine is able to destroy so much of the ozone because it acts as a catalyst. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. how ozone is destroyed by cfcs.. How Chlorine Atom Destroy Ozone.
From en.ppt-online.org
Ozone depletion online presentation How Chlorine Atom Destroy Ozone When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic”. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Topic 3 Introduction to Earth’s Atmosphere PowerPoint Presentation ID6095478 How Chlorine Atom Destroy Ozone how ozone is destroyed by cfcs. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. Ozone can be destroyed more. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Ozone is made of three oxygen atoms O 3 PowerPoint Presentation ID633298 How Chlorine Atom Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. chlorine is able to destroy so much of the ozone because it acts as a catalyst. one atom of chlorine. How Chlorine Atom Destroy Ozone.
From www.slideshare.net
What Is Ozone Layer Destruction How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). Ozone can be destroyed more. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. reactive gases containing chlorine and bromine destroy stratospheric ozone. How Chlorine Atom Destroy Ozone.
From www.meteor.iastate.edu
Manmade Chemicals, CFCs How Chlorine Atom Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. how ozone is destroyed by cfcs. Ozone can be destroyed more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). explain how chlorine and bromine atoms react with ozone which leads. How Chlorine Atom Destroy Ozone.
From slideplayer.com
OBJECTIVE 3 HOW DOES HUMAN ACTIVITY ADVERSELY AFFECT THE ATMOSPHERE? ppt download How Chlorine Atom Destroy Ozone Ozone can be destroyed more. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). how ozone is destroyed by cfcs. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made. How Chlorine Atom Destroy Ozone.
From www.sliderbase.com
Earth's Atmosphere Presentation Chemistry How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). how ozone is destroyed by cfcs. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one chlorine atom can. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Air Pollution, Climate Change, and Ozone Depletion ppt download How Chlorine Atom Destroy Ozone one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more. how ozone is destroyed by cfcs. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up. How Chlorine Atom Destroy Ozone.
From www.showme.com
How cfc's destroy ozone Science, Chemistry, Chemicalreactions ShowMe How Chlorine Atom Destroy Ozone one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. how ozone is destroyed by cfcs. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. chlorine is able to destroy so much of the ozone because it acts as a catalyst.. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Global effects Ozone depletion Greenhouse effect & Global warming. ppt download How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. reactive gases containing chlorine and bromine. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Unit 11 Ecology (Part 5) GEOCHEMICAL CYCLES and CHANGES IN ECOSYSTEMS Ms. Day AP Biology How Chlorine Atom Destroy Ozone When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). explain how chlorine and bromine atoms react with ozone which leads to the. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Ozone Depletion PowerPoint Presentation, free download ID1994903 How Chlorine Atom Destroy Ozone explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic”. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Chapter Atmosphere Section 1 Earth's Atmosphere ppt download How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. Ozone can be destroyed more. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. explain how chlorine and bromine atoms react with. How Chlorine Atom Destroy Ozone.
From funfacts.picescorp.in
CFC and Hole in the Ozone Layer How Chlorine Atom Destroy Ozone explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. chlorine is able to destroy so much of the ozone because. How Chlorine Atom Destroy Ozone.
From www.slideserve.com
PPT Chapter 13 Atmospheric Science and Air Pollution PowerPoint Presentation ID6576580 How Chlorine Atom Destroy Ozone two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. Ozone can be destroyed more. explain. How Chlorine Atom Destroy Ozone.
From www.istockphoto.com
The Ozone Layer Hole Stock Illustration Download Image Now Ozone Layer, Hole, Atmosphere How Chlorine Atom Destroy Ozone one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. chlorine is able to destroy so much of the ozone because it acts as a catalyst. how ozone is destroyed by cfcs. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). explain. How Chlorine Atom Destroy Ozone.
From slideplayer.com
The Ozone Shield ppt download How Chlorine Atom Destroy Ozone reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. one atom of chlorine can destroy more than 100,000 ozone molecules, according to the u.s. explain how chlorine and bromine atoms react with ozone which leads to. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Section 2 The Ozone Shield ppt download How Chlorine Atom Destroy Ozone explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer. reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more. how ozone is destroyed by cfcs. chlorine is able to destroy so much of the ozone because it acts as. How Chlorine Atom Destroy Ozone.
From slideplayer.com
Ozone. ppt download How Chlorine Atom Destroy Ozone one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more. When ultraviolet light waves (uv) strike cfc* (cfcl3) molecules in the upper. two scientists, mario molina and sherwood rowland, discovered that chemicals called chlorofluorocarbons (also known as cfcs). reactive gases containing chlorine and bromine destroy stratospheric. How Chlorine Atom Destroy Ozone.