Standard Enthalpy Of Formation Temperature . Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from www.youtube.com
A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
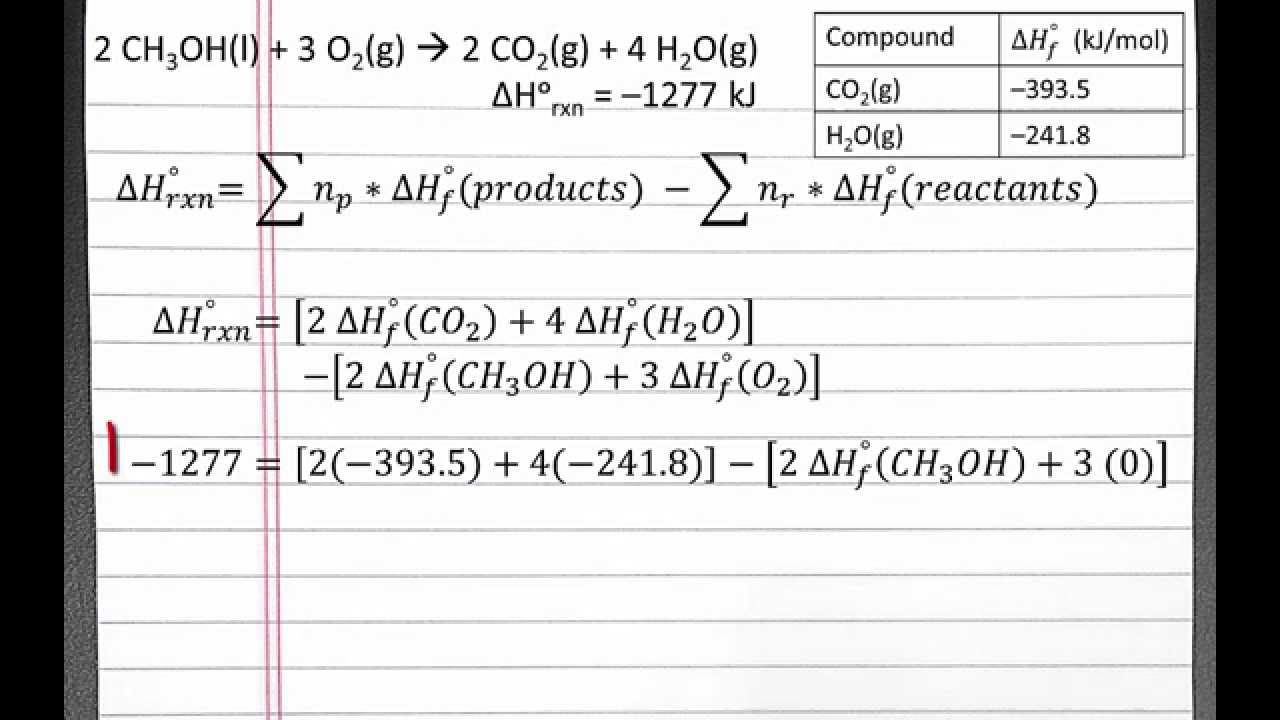
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies
Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions:
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A standard. Standard Enthalpy Of Formation Temperature.
From chemwiki.ucdavis.edu
Enthalpy Chemwiki Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound. Standard Enthalpy Of Formation Temperature.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Temperature Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Standard enthalpies of formation refer to the change. Standard Enthalpy Of Formation Temperature.
From studylib.net
Standard enthalpy of formation Standard Enthalpy Of Formation Temperature For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID2567855 Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Temperature Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with.. Standard Enthalpy Of Formation Temperature.
From slideplayer.com
THERMOCHEMISTRY Thermodynamics ppt download Standard Enthalpy Of Formation Temperature Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with.. Standard Enthalpy Of Formation Temperature.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed. Standard Enthalpy Of Formation Temperature.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation. Standard Enthalpy Of Formation Temperature.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For any substance at any particular temperature, we define the. Standard Enthalpy Of Formation Temperature.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A standard enthalpy. Standard Enthalpy Of Formation Temperature.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Temperature For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpies of. Standard Enthalpy Of Formation Temperature.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Temperature Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation refer to. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1. Standard Enthalpy Of Formation Temperature.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. A standard enthalpy of formation $δh°_f$ is an. Standard Enthalpy Of Formation Temperature.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Temperature For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation refer to the change in enthalpy that occurs when. Standard Enthalpy Of Formation Temperature.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For any substance at any particular temperature, we define the standard enthalpy of formation as the. Standard Enthalpy Of Formation Temperature.
From www.youtube.com
CHEM 101 Calculating Enthalpy of Solution YouTube Standard Enthalpy Of Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID305168 Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Temperature.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Temperature.
From www.researchgate.net
Temperature enthalpy phase diagram for steam at 1 bar. Data from Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A standard enthalpy of formation $δh°_f$ is an enthalpy. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Standard Enthalpy Of Formation Temperature For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration of 1 m. Standard Enthalpy Of Formation Temperature.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Temperature For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δh o. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Temperature.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Temperature Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed. Standard Enthalpy Of Formation Temperature.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For any substance at any particular temperature, we define the standard enthalpy of. Standard Enthalpy Of Formation Temperature.
From www.researchgate.net
Molecular weight, standard enthalpy of formation and thermal Standard Enthalpy Of Formation Temperature A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpies of formation refer to. Standard Enthalpy Of Formation Temperature.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Temperature Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration of 1 m. Standard Enthalpy Of Formation Temperature.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Of Formation Temperature 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation (\(δh^o_{f}\)). Standard Enthalpy Of Formation Temperature.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Temperature Standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID305168 Standard Enthalpy Of Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation refer to the change in enthalpy. Standard Enthalpy Of Formation Temperature.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Temperature A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration of. Standard Enthalpy Of Formation Temperature.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Temperature Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: For any substance at any particular temperature, we define the standard enthalpy of formation as the enthalpy change for a reaction in which the. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard. Standard Enthalpy Of Formation Temperature.