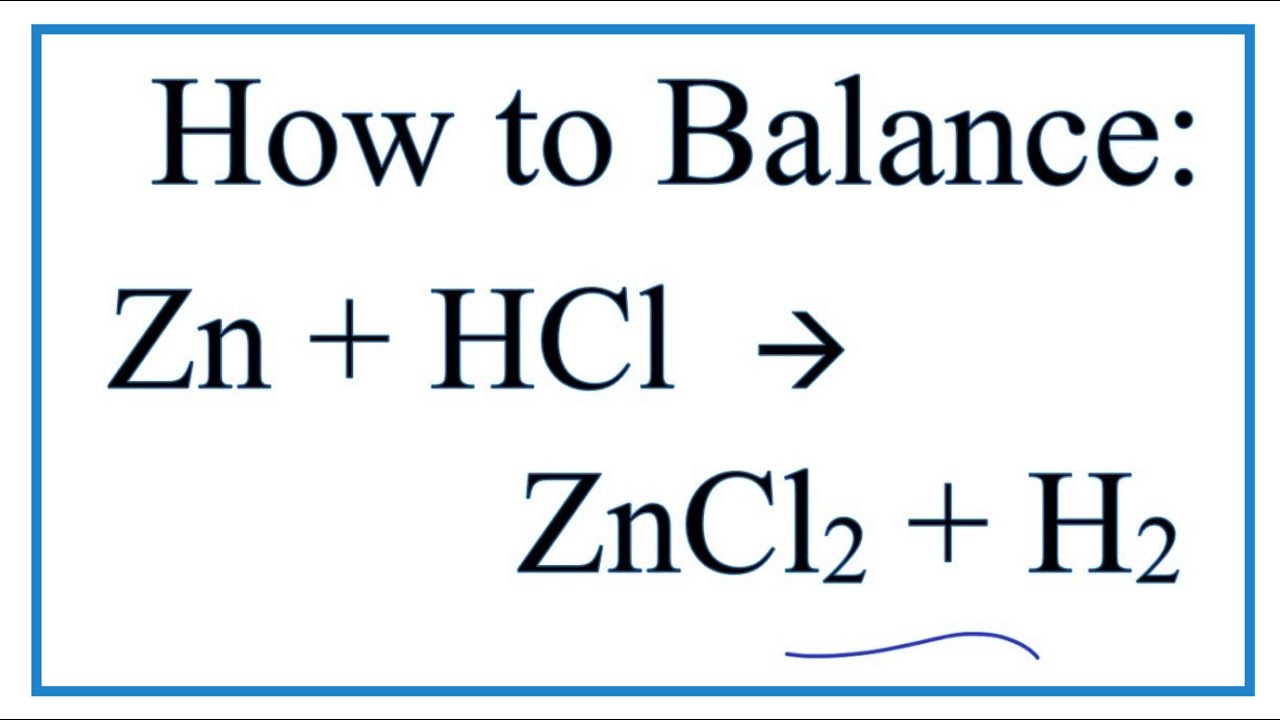
Zinc Metal And Hydrochloric Acid Net Ionic Equation . Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The balanced equation will be calculated along with the. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). In the process, hydrogen gas is produced. The reaction is given below. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. Zinc is oxidized by hydrochloric acid to form zinc chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! To balance the equation given to you, multiply the hydrochloric acid by 2. In this instance, the species undergoing change are zinc metal and hydrogen.
from shotprofessional22.gitlab.io
Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The balanced equation will be calculated along with the. In the process, hydrogen gas is produced. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Zinc is oxidized by hydrochloric acid to form zinc chloride. To balance the equation given to you, multiply the hydrochloric acid by 2. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The reaction is given below. Enter an equation of an ionic chemical equation and press the balance button.
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas
Zinc Metal And Hydrochloric Acid Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. To balance the equation given to you, multiply the hydrochloric acid by 2. Enter an equation of an ionic chemical equation and press the balance button. Zinc is oxidized by hydrochloric acid to form zinc chloride. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The balanced equation will be calculated along with the. In this instance, the species undergoing change are zinc metal and hydrogen. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The reaction is given below. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. In the process, hydrogen gas is produced.
From treatybottle13.pythonanywhere.com
Divine Complete And Net Ionic Equations Calculator Aqa Gcse Physics Zinc Metal And Hydrochloric Acid Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. The reaction is given below. Enter an equation of an ionic chemical equation and press the balance button. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. To balance the equation given to you, multiply the hydrochloric acid by. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From dxousntek.blob.core.windows.net
What Happens If You Combine Zinc And Hydrochloric Acid at Debrah Sankey Zinc Metal And Hydrochloric Acid Net Ionic Equation To balance the equation given to you, multiply the hydrochloric acid by 2. In this instance, the species undergoing change are zinc metal and hydrogen. Zinc is oxidized by hydrochloric acid to form zinc chloride. Enter an equation of an ionic chemical equation and press the balance button. There are three main steps for writing the net ionic equation for. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Zinc Metal And Hydrochloric Acid Net Ionic Equation To balance the equation given to you, multiply the hydrochloric acid by 2. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. The balanced equation will be calculated along with the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! In this instance, the species undergoing change. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate And Hydrobromic Acid Net Ionic Equation Tessshebaylo Zinc Metal And Hydrochloric Acid Net Ionic Equation It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.youtube.com
NaOH + HCl Sodium Hydroxide & Hydrochloric Acid Net Ionic Equation Zinc Metal And Hydrochloric Acid Net Ionic Equation The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The reaction is given below. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. The balanced equation will be calculated along with the.. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced equilibrium chemical equation for Sodium Zinc Metal And Hydrochloric Acid Net Ionic Equation There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). To balance the equation given to you, multiply the hydrochloric acid by 2. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The net ionic equation is. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Zinc Metal And Hydrochloric Acid Net Ionic Equation It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The reaction is given below. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. Zinc is oxidized by hydrochloric acid to form zinc chloride. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). In the process, hydrogen gas is produced. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. There are three main steps for writing the net ionic equation for zn + hcl = zncl2. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From hedycabrera.blogspot.com
View Calcium Hydroxide And Hydrochloric Acid Reaction US Zinc Metal And Hydrochloric Acid Net Ionic Equation It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the balanced equation that shows only the species that. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Metal And Hydrochloric Acid Net Ionic Equation There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. Enter an equation of an ionic chemical equation and press the balance button. In the process, hydrogen gas is produced. Zn(s) +2hcl(aq). Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc Metal And Hydrochloric Acid Net Ionic Equation The net ionic equation is the balanced equation that shows only the species that undergo chemical change. To balance the equation given to you, multiply the hydrochloric acid by 2. Zinc is oxidized by hydrochloric acid to form zinc chloride. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From dejameowhuff.blogspot.com
Sodium Acetate and Hydrochloric Acid Net Ionic Equation Zinc Metal And Hydrochloric Acid Net Ionic Equation In this instance, the species undergoing change are zinc metal and hydrogen. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! In the process, hydrogen. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Metal And Hydrochloric Acid Net Ionic Equation Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. To balance the equation given to you, multiply the hydrochloric acid by 2. In the process, hydrogen gas is produced. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! Enter an equation. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. Enter an equation of an ionic chemical equation and press the balance button. The reaction is given below. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! To balance the equation given to you, multiply the hydrochloric. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. To balance the equation given to you, multiply the hydrochloric acid by 2. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. In this instance, the species undergoing change are zinc metal and hydrogen. In the process, hydrogen gas is produced. Zinc is. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate And Hydrochloric Acid Net Ionic Equation Tessshebaylo Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Zinc is oxidized by hydrochloric acid to form zinc chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tessshebaylo.com
Sodium Bicarbonate And Hydrochloric Acid Net Ionic Equation Tessshebaylo Zinc Metal And Hydrochloric Acid Net Ionic Equation The bubbles are hydrogen gas (right side of equation \ref{eq:1}). There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. Enter an equation of an ionic chemical equation and press the balance button. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. To. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HCl = ZnCl2 + H2 YouTube Zinc Metal And Hydrochloric Acid Net Ionic Equation The net ionic equation is the balanced equation that shows only the species that undergo chemical change. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The balanced equation will be calculated along with the. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. To. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate And Hydrobromic Acid Net Ionic Equation Tessshebaylo Zinc Metal And Hydrochloric Acid Net Ionic Equation Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. In the process, hydrogen gas is produced. There are three main steps for writing the net ionic equation for zn + hcl = zncl2. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.tessshebaylo.com
What Is The Net Ionic Equation For Reaction Of Hydrochloric Acid With Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. The reaction is given below. To balance the equation given to you, multiply the hydrochloric acid by 2. Zinc is oxidized by hydrochloric acid to form zinc chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Metal And Hydrochloric Acid Net Ionic Equation The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. In this instance, the species undergoing change are zinc metal and hydrogen. The reaction is given below. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. Enter an equation of an. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). In this instance, the species undergoing change are zinc metal and hydrogen. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! There are three main steps for writing the net ionic equation for zn + hcl = zncl2. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From koltenfersrubio.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Zinc Metal And Hydrochloric Acid Net Ionic Equation Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). In this instance, the species undergoing change are zinc metal and hydrogen. The balanced equation will be calculated along with the. Zn(s). Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From dejameowhuff.blogspot.com
Sodium Acetate and Hydrochloric Acid Net Ionic Equation Zinc Metal And Hydrochloric Acid Net Ionic Equation The reaction is given below. In this instance, the species undergoing change are zinc metal and hydrogen. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. It is fairly obvious that. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From studylib.net
Net Ionic Equations Zinc Metal And Hydrochloric Acid Net Ionic Equation The reaction is given below. To balance the equation given to you, multiply the hydrochloric acid by 2. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). The balanced equation will be calculated along with the. Zinc is oxidized by hydrochloric acid to form zinc chloride. There are three main steps for writing the net ionic equation for zn. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Metal And Hydrochloric Acid Net Ionic Equation Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. The reaction is given below. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The net ionic equation. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From estrellatuhooper.blogspot.com
Calcium Chloride and Hydrochloric Acid Balanced Equation EstrellatuHooper Zinc Metal And Hydrochloric Acid Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. To balance the equation given to you, multiply the hydrochloric acid by 2. There are three main steps for writing the net ionic equation for zn + hcl = zncl2. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.numerade.com
SOLVED 'Hydrochloric Acid Sodium Hydroxide no rra(tiun lirav Zinc Metal And Hydrochloric Acid Net Ionic Equation In the process, hydrogen gas is produced. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. The bubbles are hydrogen gas (right side of equation \ref{eq:1}). Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. Enter an equation of an ionic chemical equation and. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From dxorsdznx.blob.core.windows.net
Zinc And Hydrochloric Acid Net Ionic Equation at Kristine Murray blog Zinc Metal And Hydrochloric Acid Net Ionic Equation The reaction is given below. Enter an equation of an ionic chemical equation and press the balance button. It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! In the process, hydrogen gas is produced. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. In this instance, the species undergoing change are zinc metal and hydrogen. The net ionic. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.numerade.com
SOLVED (3) HCI + NaOH In Part I An aqueous solution of sodium Zinc Metal And Hydrochloric Acid Net Ionic Equation In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. In this instance, the species undergoing change are zinc metal and hydrogen. The net ionic equation is the balanced equation that shows only the species that undergo chemical change. Enter an equation of an ionic chemical equation and press the balance button. The. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From socratic.org
What is the chemical equation for HCl dissolving into water and Zinc Metal And Hydrochloric Acid Net Ionic Equation Zinc is oxidized by hydrochloric acid to form zinc chloride. In this instance, the species undergoing change are zinc metal and hydrogen. To balance the equation given to you, multiply the hydrochloric acid by 2. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. The bubbles are hydrogen. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.reddit.com
Write the net ionic equation in which the slightly soluble salt Zinc Metal And Hydrochloric Acid Net Ionic Equation The balanced equation will be calculated along with the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. In this instance, the species undergoing change are zinc metal and hydrogen. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. The reaction is given below. There are three main steps for writing the net ionic equation for zn + hcl. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From boys.velvet.jp
Net Ionic Equation For Na2CO3 HCl Sodium Carbonate, 42 OFF Zinc Metal And Hydrochloric Acid Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions. There are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. In this instance, the species undergoing. Zinc Metal And Hydrochloric Acid Net Ionic Equation.
From www.chegg.com
Solved Write a net ionic equation for the reaction that Zinc Metal And Hydrochloric Acid Net Ionic Equation To balance the equation given to you, multiply the hydrochloric acid by 2. The reaction is given below. Zn(s) +2hcl(aq) → zncl2(aq) +h2(g) you know that. Zinc is oxidized by hydrochloric acid to form zinc chloride. In this instance, the species undergoing change are zinc metal and hydrogen. The balanced equation will be calculated along with the. Oxidation/reduction, gas forming. Zinc Metal And Hydrochloric Acid Net Ionic Equation.