Suspended Colloidal Particles . Colloid particles are much smaller than suspension particles. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Everyday examples of suspensions include mud, flour in. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. A suspension is a heterogeneous mixture that settles out upon standing. This book addresses the properties of particles in colloidal suspensions. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. The main difference between colloid and suspension lies in the size of particles. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles separate from the. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing.
from www.myshared.ru
A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture that settles out upon standing. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. The dispersed particles separate from the. This book addresses the properties of particles in colloidal suspensions. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. Colloid particles are much smaller than suspension particles. Everyday examples of suspensions include mud, flour in.
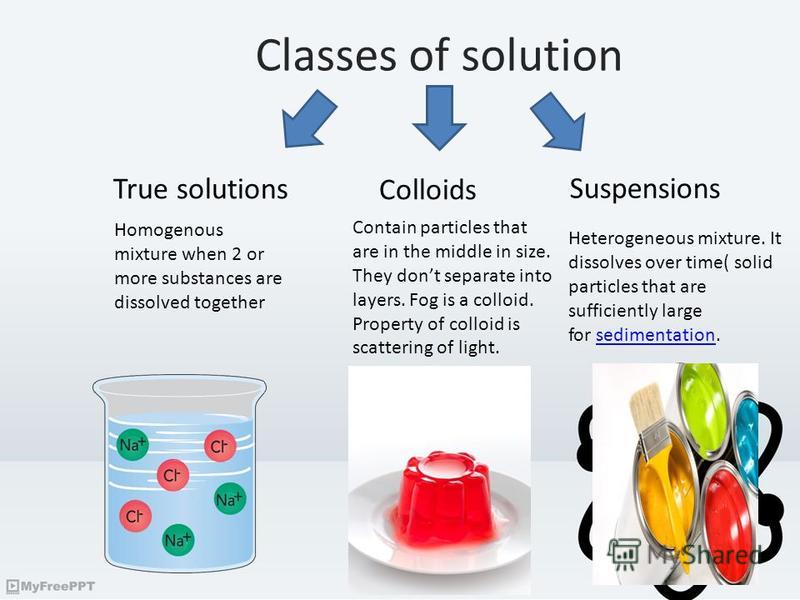
Презентация на тему "Colloidal systems. Classes of solution True
Suspended Colloidal Particles Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Colloid particles are much smaller than suspension particles. The dispersed particles separate from the. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Everyday examples of suspensions include mud, flour in. A suspension is a heterogeneous mixture that settles out upon standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. This book addresses the properties of particles in colloidal suspensions. The main difference between colloid and suspension lies in the size of particles.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The dispersed particles separate from the. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along. Suspended Colloidal Particles.
From www.vecteezy.com
Coagulation of Colloid Particles vector illustration 21669350 Vector Suspended Colloidal Particles Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are much smaller than suspension particles. The dispersed particles separate from the. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Because. Suspended Colloidal Particles.
From www.researchgate.net
Schematic illustration of a colloidal suspension of silica spheres in Suspended Colloidal Particles The main difference between colloid and suspension lies in the size of particles. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Everyday examples of suspensions include mud, flour in. A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are much smaller. Suspended Colloidal Particles.
From pubs.acs.org
Colloidal Particles at a Range of FluidFluid Interfaces Langmuir Suspended Colloidal Particles Everyday examples of suspensions include mud, flour in. The dispersed particles separate from the. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions,. Suspended Colloidal Particles.
From www.geeksforgeeks.org
What is a Suspension? Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Colloid particles are much smaller than suspension particles. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. This book addresses the properties of particles in colloidal suspensions.. Suspended Colloidal Particles.
From www.science.org
Colloidal matter Packing, geometry, and entropy Science Suspended Colloidal Particles A suspension is a heterogeneous mixture that settles out upon standing. The main difference between colloid and suspension lies in the size of particles. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. This book addresses the properties of particles in colloidal suspensions. Colloid particles are much smaller than suspension particles. Here is how. Suspended Colloidal Particles.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspended Colloidal Particles A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; This book addresses the properties of particles in colloidal suspensions. The dispersed particles separate from the. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions. Suspended Colloidal Particles.
From www.researchgate.net
Backscattered intensity from gold colloidal particles suspended in Suspended Colloidal Particles The dispersed particles separate from the. The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. This book addresses the properties of. Suspended Colloidal Particles.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspended Colloidal Particles Colloid particles are much smaller than suspension particles. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. This book addresses the properties of particles in colloidal suspensions. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Everyday examples of suspensions include mud, flour. Suspended Colloidal Particles.
From www.researchgate.net
Mechanism of colloidal PCs assembly. (A) Colloidal particles assembly Suspended Colloidal Particles A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Everyday examples of suspensions include mud, flour in. The main difference between colloid and suspension lies in the size of particles. Because the dispersed particles of a colloid are. Suspended Colloidal Particles.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspended Colloidal Particles Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are much smaller than suspension particles. This book addresses the properties of particles in colloidal. Suspended Colloidal Particles.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Suspended Colloidal Particles Colloid particles are much smaller than suspension particles. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. This book addresses the properties of particles in colloidal suspensions. Everyday examples of suspensions include mud, flour in. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A suspension is. Suspended Colloidal Particles.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Suspended Colloidal Particles As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Everyday examples of suspensions include mud, flour in. Colloid particles are much smaller than suspension particles. It has a focus on particle aggregates and the dependency of their physical. Suspended Colloidal Particles.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspended Colloidal Particles A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A suspension is a heterogeneous mixture that settles out upon standing. This book addresses the properties of particles in colloidal suspensions. The dispersed particles separate from the. Everyday examples of suspensions include mud, flour in. Here is how to distinguish among solutions, suspensions, colloids,. Suspended Colloidal Particles.
From www.researchgate.net
Colloidal particles in an stabilizing electrolyte. (a) Until the Suspended Colloidal Particles The main difference between colloid and suspension lies in the size of particles. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. This book addresses the properties of particles in colloidal suspensions. A suspension is a heterogeneous mixture. Suspended Colloidal Particles.
From www.nagwa.com
Vidéo de la leçon Colloïdes et les suspensions Nagwa Suspended Colloidal Particles As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. The dispersed particles separate from the. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological.. Suspended Colloidal Particles.
From www.pinterest.com
Solutions, Colloids, Suspensions Science lessons, Chemistry lessons Suspended Colloidal Particles It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension. Suspended Colloidal Particles.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Suspended Colloidal Particles Everyday examples of suspensions include mud, flour in. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is a heterogeneous mixture that settles out upon standing. The main difference between colloid and. Suspended Colloidal Particles.
From surfguppy.com
Properties of Colloids Surfguppy Chemistry made easy visual learning Suspended Colloidal Particles A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; This book addresses the properties of particles in colloidal suspensions. Everyday examples of suspensions include mud, flour in. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Colloid particles are much smaller than suspension particles. Because the dispersed. Suspended Colloidal Particles.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspended Colloidal Particles As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in. This book addresses the properties of particles in colloidal suspensions. The main difference between colloid and suspension lies in the size of particles. It has a. Suspended Colloidal Particles.
From pubs.acs.org
Dependent Scattering in Thick and Concentrated Colloidal Suspensions Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Colloid particles are much smaller than suspension particles. As a consequence, dirt particles become suspended as colloidal particles and are readily. Suspended Colloidal Particles.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; The dispersed. Suspended Colloidal Particles.
From collegedunia.com
Classification of Colloids Properties & Phases Suspended Colloidal Particles Everyday examples of suspensions include mud, flour in. The dispersed particles separate from the. Colloid particles are much smaller than suspension particles. This book addresses the properties of particles in colloidal suspensions. A suspension is a heterogeneous mixture that settles out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples. Suspended Colloidal Particles.
From www.youtube.com
Types of Mixtures Solution, Suspension, Colloid NOTES YouTube Suspended Colloidal Particles A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. A suspension is a heterogeneous mixture that settles out upon standing. The main difference between colloid and suspension lies in the. Suspended Colloidal Particles.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Suspended Colloidal Particles This book addresses the properties of particles in colloidal suspensions. Colloid particles are much smaller than suspension particles. Everyday examples of suspensions include mud, flour in. The dispersed particles separate from the. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Because the dispersed particles of a colloid are not as large as those. Suspended Colloidal Particles.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspended Colloidal Particles The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture that settles out upon standing. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Everyday examples of suspensions include mud, flour in. This book addresses the properties of particles in colloidal suspensions. Here is how. Suspended Colloidal Particles.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspended Colloidal Particles A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in. As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. Colloid particles are much smaller than suspension particles. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of. Suspended Colloidal Particles.
From fyohcljfj.blob.core.windows.net
Colloidal Solid Suspended In Liquid at Cynthia Phillips blog Suspended Colloidal Particles It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. This book. Suspended Colloidal Particles.
From www.britannica.com
Brownian motion in a colloid Britannica Suspended Colloidal Particles The dispersed particles separate from the. This book addresses the properties of particles in colloidal suspensions. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The main difference between colloid and suspension lies in. Suspended Colloidal Particles.
From www.pharmaguideline.com
Colloidal dispersions Classification of dispersed systems & their Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Colloid particles are much smaller than suspension particles. This book addresses the properties of particles in colloidal suspensions. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each.. Suspended Colloidal Particles.
From wiki.anton-paar.com
The influence of particles on suspension rheology Anton Paar Wiki Suspended Colloidal Particles Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The main difference between colloid and suspension lies in the size of particles. It has a focus on particle aggregates and the dependency of their physical behaviour on morphological. This book addresses the properties of particles in. Suspended Colloidal Particles.
From courses.lumenlearning.com
Colloids Chemistry Atoms First Suspended Colloidal Particles Colloid particles are much smaller than suspension particles. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles separate from the. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The main difference between. Suspended Colloidal Particles.
From membraneworks.com.au
Colloidal fouling (clay, iron and organic materials) Membrane Works Suspended Colloidal Particles Colloid particles are much smaller than suspension particles. This book addresses the properties of particles in colloidal suspensions. The dispersed particles separate from the. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in. Suspended Colloidal Particles.
From courses.lumenlearning.com
Colloids Chemistry Suspended Colloidal Particles As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. The dispersed particles separate from the. Colloid particles are much smaller than suspension particles. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; It has a focus on particle aggregates and the dependency of their physical behaviour on. Suspended Colloidal Particles.
From www.shutterstock.com
218 vectores de Colloid Vectores, imágenes y arte vectorial de stock Suspended Colloidal Particles As a consequence, dirt particles become suspended as colloidal particles and are readily washed away. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Everyday examples of suspensions include mud, flour in. The main difference between colloid and suspension lies in the size of particles. Here is how to distinguish among solutions, suspensions,. Suspended Colloidal Particles.