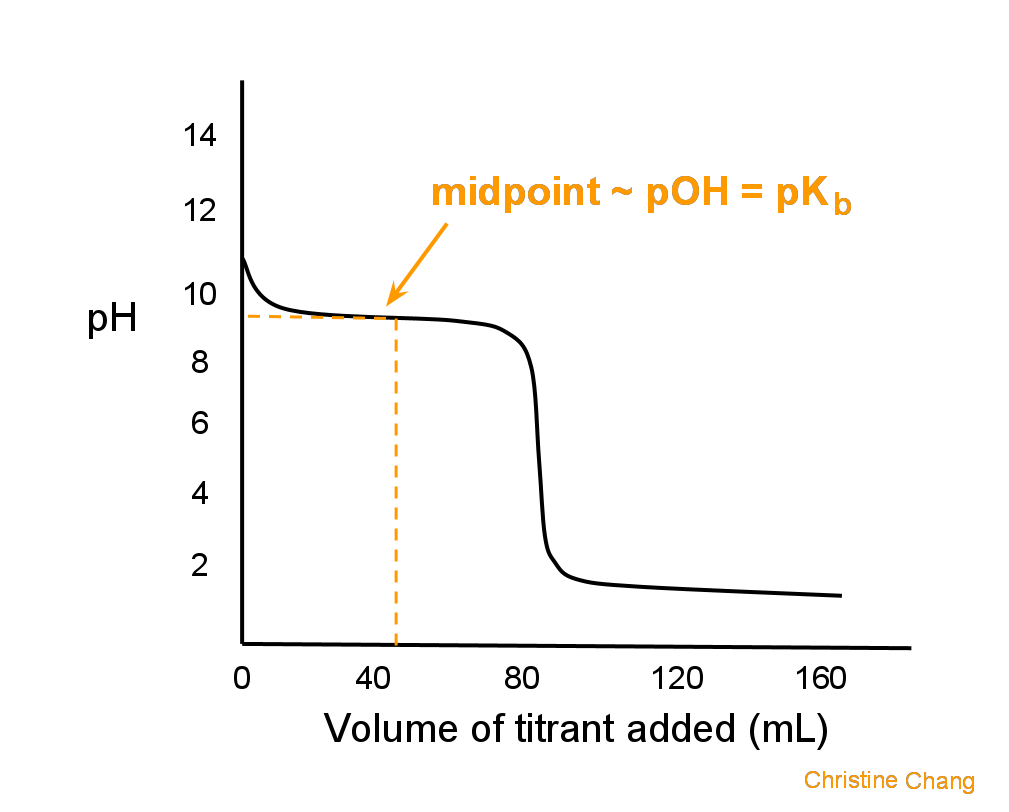
Titration Curve Points . Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The ph does not change in a regular manner as the acid is added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent.
from chem.libretexts.org
The ph does not change in a regular manner as the acid is added. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph.
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Titration Curve Points The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The ph does not change in a regular manner as the acid is added.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. Titration curves show how the ph of an acidic or basic solution changes as a. Titration Curve Points.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Points The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The. Titration Curve Points.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Points A titration curve is a graphical representation of the ph of a solution during a titration. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. Each curve has horizontal sections where a lot of bases can be added without changing the ph. Titration Curve Points.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Points A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The end point of a titration is the point at which an indicator changes. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The. Titration Curve Points.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Points A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The ph does not change in a regular manner as the acid is added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. Titration Curve Points.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Points The end point of a titration is the point at which an indicator changes. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on. Titration Curve Points.
From studylib.net
Titration Curve weak base with strong acid START Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a. Titration Curve Points.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Curve Points The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The ph does not change in a regular manner as the acid is added. A titration curve is a graphical representation of the. Titration Curve Points.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Points The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A titration curve. Titration Curve Points.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Points A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The ph does not change in a regular manner as the acid is added. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often. Titration Curve Points.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A ph curve. Titration Curve Points.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curve Points Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result. Titration Curve Points.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Points The ph does not change in a regular manner as the acid is added. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the. Titration Curve Points.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve Points A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The ph does not change in a regular manner as the acid is added. Each curve has horizontal sections where a lot of bases. Titration Curve Points.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Points The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The ph does not change in a regular manner as the acid is added. A ph curve is a graph showing how the ph of a solution changes as the. Titration Curve Points.
From www.jove.com
AcidBase Titration Curves JoVE Titration Curve Points Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The ph does not change in a regular manner as the acid is added. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the. Titration Curve Points.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Points A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. The ph does not change in a regular manner as the acid is added. Each curve has horizontal sections where a lot of bases can be added without changing the. Titration Curve Points.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration Curve Points The end point of a titration is the point at which an indicator changes. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The ph does not change in a regular manner as the acid is added. A titration curve is a graphical representation of the ph of a. Titration Curve Points.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Points A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The ph does not change in a regular manner as the acid is added. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration. Titration Curve Points.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Points Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. The ph does not change in a regular manner as. Titration Curve Points.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Points Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The ph does not change in a regular manner as the acid is added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point. Titration Curve Points.
From www.youtube.com
Titration Curve for glycine in a easiest way YouTube Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how. Titration Curve Points.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Points The ph does not change in a regular manner as the acid is added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. A ph curve is a graph showing. Titration Curve Points.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Points Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. Titration Curve Points.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Points A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph. Titration Curve Points.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Points The ph does not change in a regular manner as the acid is added. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic. Titration Curve Points.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. Titration Curve Points.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Curve Points Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. Each curve has horizontal sections where a. Titration Curve Points.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Points A titration curve is a graphical representation of the ph of a solution during a titration. The ph does not change in a regular manner as the acid is added. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the. Titration Curve Points.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes. The ph does not change. Titration Curve Points.
From mavink.com
H2so4 Titration Curve Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The end point of a titration is the point at which an indicator changes. A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. A. Titration Curve Points.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Points A ph curve is a graph showing how the ph of a solution changes as the acid (or base) is added the result is characteristically shaped graph. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. A titration curve is a graphical representation of the ph of a solution during a. Titration Curve Points.
From mungfali.com
Titration Curve Labeled Titration Curve Points Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The end point of a titration is the point at which an indicator changes. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A ph curve is a graph showing how. Titration Curve Points.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curve Points The ph does not change in a regular manner as the acid is added. The end point of a titration is the point at which an indicator changes. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. A ph curve is a graph showing how the ph of a. Titration Curve Points.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Points The end point of a titration is the point at which an indicator changes. Each curve has horizontal sections where a lot of bases can be added without changing the ph much. The ph does not change in a regular manner as the acid is added. Titration curves show how the ph of an acidic or basic solution changes as. Titration Curve Points.