Titration To Determine Zinc Concentration . For example, as shown in figure 9.35, we can determine the. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. Zinc is an example of a metal ion that is titrated in acidic solution. You can perform a zinc determination using this titration method with methyl orange as your indicator. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. The metals that react more weakly with edta must be. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should.
from www.chegg.com
From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. The metals that react more weakly with edta must be. You can perform a zinc determination using this titration method with methyl orange as your indicator. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. Zinc is an example of a metal ion that is titrated in acidic solution. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. For example, as shown in figure 9.35, we can determine the.
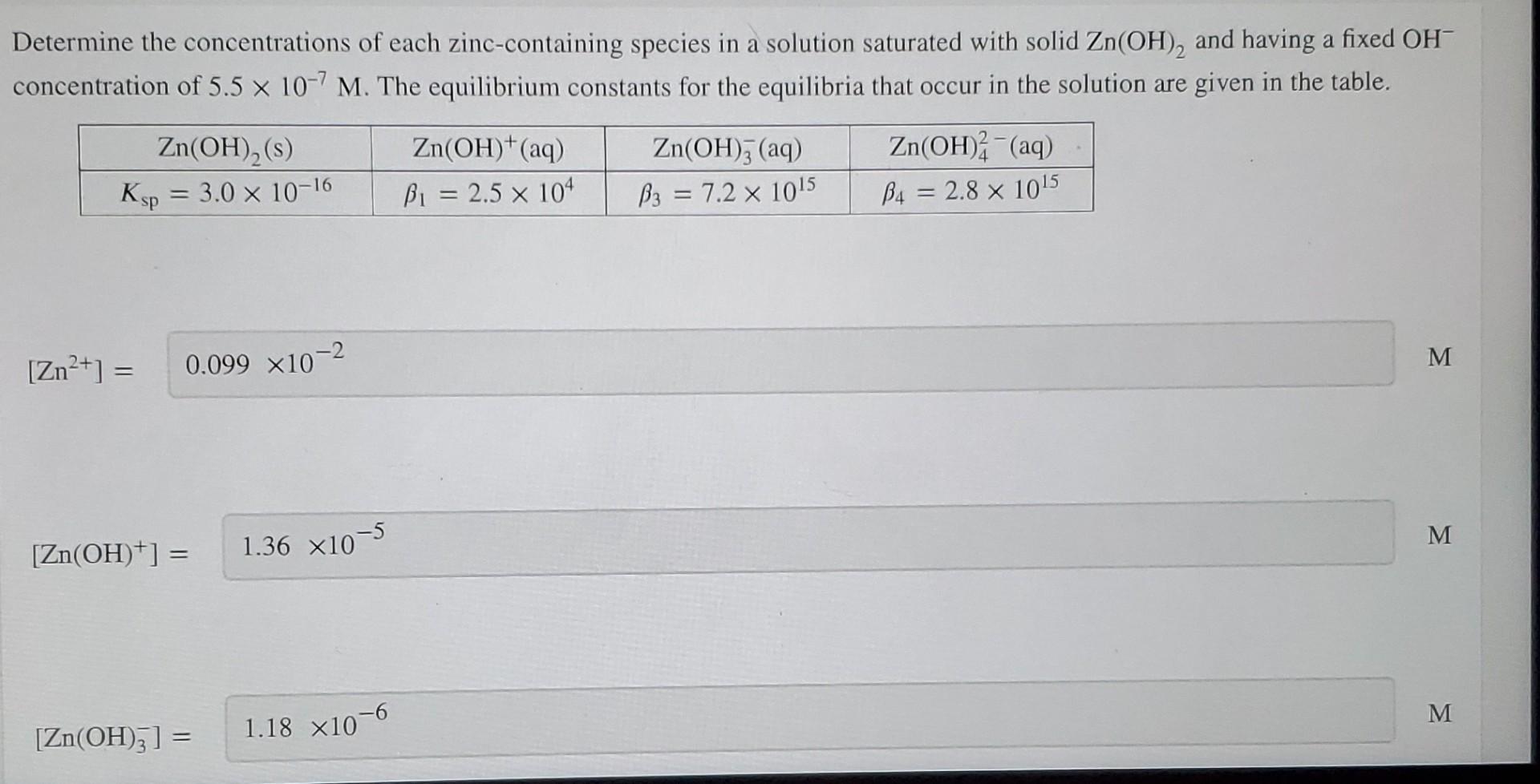
Solved Determine the concentrations of each zinccontaining
Titration To Determine Zinc Concentration Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Zinc is an example of a metal ion that is titrated in acidic solution. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. The metals that react more weakly with edta must be. You can perform a zinc determination using this titration method with methyl orange as your indicator. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. For example, as shown in figure 9.35, we can determine the.
From www.researchgate.net
Relation between plasma zinc concentration and fractional zinc Titration To Determine Zinc Concentration The metals that react more weakly with edta must be. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. You can perform a zinc determination using this titration method with methyl orange as your indicator. For example, as shown in figure 9.35, we can determine the. Titer determination of edta 0.1 mol/l with zinc sulfate. Titration To Determine Zinc Concentration.
From www.researchgate.net
ITC data of zincbinding to whey proteins. Titration curves of Titration To Determine Zinc Concentration The metals that react more weakly with edta must be. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. For example, as. Titration To Determine Zinc Concentration.
From www.chegg.com
Solved Titration to Determine the Empirical Formula of Zinc Titration To Determine Zinc Concentration While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. You can perform a zinc determination using this titration method with methyl orange as your indicator. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc.. Titration To Determine Zinc Concentration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Zinc is an example of a metal ion that is titrated in acidic solution. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results. Titration To Determine Zinc Concentration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration To Determine Zinc Concentration Zinc is an example of a metal ion that is titrated in acidic solution. You can perform a zinc determination using this titration method with methyl orange as your indicator. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Titer determination of edta. Titration To Determine Zinc Concentration.
From www.researchgate.net
9 Effect of changing zinc concentration and alloying elements on the Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination. Titration To Determine Zinc Concentration.
From www.researchgate.net
Dosed zinc concentration versus measured zinc concentration in pore Titration To Determine Zinc Concentration Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. The metals that react more weakly with edta must be. You can perform a zinc determination using this titration method with methyl orange. Titration To Determine Zinc Concentration.
From www.scribd.com
Determination of Zinc by Titration PDF Titration Chemistry Titration To Determine Zinc Concentration While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. For example, as shown in figure 9.35, we can determine the. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. From the known stoichiometry of the reaction between the titrant and the analyte, one. Titration To Determine Zinc Concentration.
From www.researchgate.net
Relationship between equilibrium zinc concentration and its uptake at Titration To Determine Zinc Concentration The metals that react more weakly with edta must be. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Zinc is an example of a metal ion that is. Titration To Determine Zinc Concentration.
From www.academia.edu
(DOC) DETERMINATION OF ZINC BY TITRATION tare deinne Academia.edu Titration To Determine Zinc Concentration For example, as shown in figure 9.35, we can determine the. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc.. Titration To Determine Zinc Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration To Determine Zinc Concentration Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. For example, as shown in figure 9.35, we can determine the. You can perform a zinc determination using this titration method with methyl orange as. Titration To Determine Zinc Concentration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Titration To Determine Zinc Concentration A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. The metals that react more weakly with edta must be. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. While it is possible to achieve relatively good results by titration with edta. Titration To Determine Zinc Concentration.
From www.chegg.com
Titration to Determine the Empirical Formula of Zinc Titration To Determine Zinc Concentration You can perform a zinc determination using this titration method with methyl orange as your indicator. Zinc is an example of a metal ion that is titrated in acidic solution. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. Spectrograms of both standard and sample solutions of zinc. Titration To Determine Zinc Concentration.
From www.youtube.com
How to do a titration and calculate the concentration YouTube Titration To Determine Zinc Concentration A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination. Titration To Determine Zinc Concentration.
From www.chegg.com
Solved Titration to Determine the Empirical Formula of Zinc Titration To Determine Zinc Concentration For example, as shown in figure 9.35, we can determine the. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Zinc is an example of a metal ion that is titrated in acidic solution.. Titration To Determine Zinc Concentration.
From www.researchgate.net
Results for the photometric titration of zinc and copper mixtures at pH Titration To Determine Zinc Concentration A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. For example, as shown in figure 9.35, we can determine the. The metals that react more weakly with edta must be. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. While it is possible. Titration To Determine Zinc Concentration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration To Determine Zinc Concentration Zinc is an example of a metal ion that is titrated in acidic solution. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore. Titration To Determine Zinc Concentration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Zinc is an example of a metal ion that is titrated in acidic solution. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with. Titration To Determine Zinc Concentration.
From www.researchgate.net
Titration of imidazole binding to zinc(II) porphyrin 1ZnO 2. Inset is Titration To Determine Zinc Concentration A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. The metals. Titration To Determine Zinc Concentration.
From www.researchgate.net
Relation between plasma zinc concentration and fractional zinc Titration To Determine Zinc Concentration The metals that react more weakly with edta must be. You can perform a zinc determination using this titration method with methyl orange as your indicator. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. Zinc is an example of a metal ion that is titrated in acidic. Titration To Determine Zinc Concentration.
From www.scribd.com
Determination of Zinc Ion Concentration Through Complexometric Titration To Determine Zinc Concentration Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. For example, as shown in figure 9.35, we can determine the. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. While it is possible to. Titration To Determine Zinc Concentration.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration To Determine Zinc Concentration While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. The metals that react more weakly with edta must be. Zinc is an example of a metal ion that is titrated in acidic solution. Spectrograms. Titration To Determine Zinc Concentration.
From www.chegg.com
Solved Determine the concentrations of each zinccontaining Titration To Determine Zinc Concentration For example, as shown in figure 9.35, we can determine the. Zinc is an example of a metal ion that is titrated in acidic solution. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. The metals that react more weakly with edta must be. Titer determination. Titration To Determine Zinc Concentration.
From www.researchgate.net
Concentration of zinc, Zn Download Table Titration To Determine Zinc Concentration Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. For example, as shown in figure 9.35, we can determine the. From the known stoichiometry of the reaction between the titrant and the. Titration To Determine Zinc Concentration.
From www.chegg.com
Solved How to calculate the mass of zinc chloride titrated, Titration To Determine Zinc Concentration For example, as shown in figure 9.35, we can determine the. The metals that react more weakly with edta must be. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta. Titration To Determine Zinc Concentration.
From www.youtube.com
Estimation of Zinc in Brass ‖ Complexometric Titration ‖ Combined Geo Titration To Determine Zinc Concentration Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. You can perform a zinc determination using this titration method with methyl orange as your indicator. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc.. Titration To Determine Zinc Concentration.
From www.chegg.com
Solved Determine the concentrations of each zinccontaining Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. For example, as shown in figure 9.35, we can determine the. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc.. Titration To Determine Zinc Concentration.
From studylib.net
Complexometric Titration of Zinc Titration To Determine Zinc Concentration While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. For example, as shown in figure 9.35, we can determine the. The metals that react more weakly with. Titration To Determine Zinc Concentration.
From theedge.com.hk
Chemistry How To Titration The Edge Titration To Determine Zinc Concentration Zinc is an example of a metal ion that is titrated in acidic solution. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph 10 with zinc. The metals that react more weakly with edta must be. From the known stoichiometry of the reaction between the titrant and the analyte, one can. Titration To Determine Zinc Concentration.
From www.alamy.com
Titration experiment. Students titrating a reaction mixture. Titration Titration To Determine Zinc Concentration A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. You can perform a zinc determination using this titration method with methyl orange as your indicator. The metals that react more weakly with edta must be. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination. Titration To Determine Zinc Concentration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration To Determine Zinc Concentration While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Zinc is an example of a metal ion that is titrated in acidic solution. From the known stoichiometry of the reaction between the titrant and. Titration To Determine Zinc Concentration.
From www.aplustopper.com
How does titration determine concentration? A Plus Topper Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. The metals that react more weakly with edta must be. You can perform a zinc determination using this titration method with methyl orange as your indicator. Titer determination of edta 0.1 mol/l with zinc. Titration To Determine Zinc Concentration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration To Determine Zinc Concentration You can perform a zinc determination using this titration method with methyl orange as your indicator. From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. Titer determination of edta 0.1 mol/l with zinc sulfate titer determination of edta by complexometric titration at ph. Titration To Determine Zinc Concentration.
From pubs.acs.org
Determination of Zinc Oxide in Pharmaceutical Preparations by EDTA Titration To Determine Zinc Concentration From the known stoichiometry of the reaction between the titrant and the analyte, one can calculate the mols of the analyte and therefore the mass and/or. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. Zinc is an example of a metal ion that is titrated in acidic. Titration To Determine Zinc Concentration.
From www.chegg.com
Titration to Determine the Empirical Formula of Zinc Titration To Determine Zinc Concentration For example, as shown in figure 9.35, we can determine the. While it is possible to achieve relatively good results by titration with edta prepared directly from the solid, better results should. A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the. Titration To Determine Zinc Concentration.