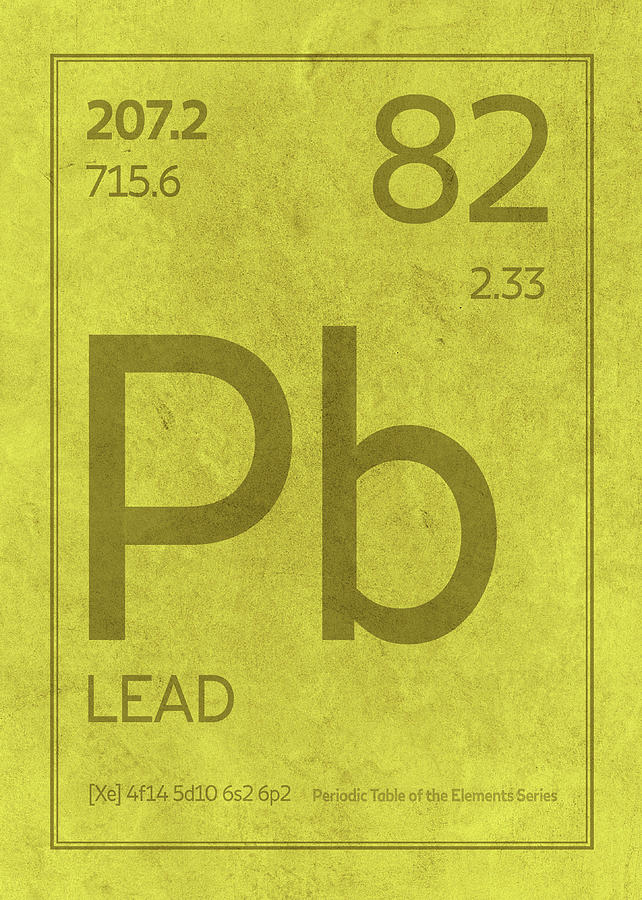
Lead Pb Charge . When a lead atom loses two electrons it exhibits a +2 charge. 93 rows this table shows the most common charges for atoms of the chemical elements. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.
from pixels.com
For example, iron(ii) has a. 93 rows this table shows the most common charges for atoms of the chemical elements. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital.
Lead Pb Element Symbol Periodic Table Series 082 Mixed Media by Design
Lead Pb Charge When a lead atom loses two electrons it exhibits a +2 charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When a lead atom loses two electrons it exhibits a +2 charge. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. 93 rows this table shows the most common charges for atoms of the chemical elements. For example, iron(ii) has a. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+).
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. 93 rows. Lead Pb Charge.
From utedzz.blogspot.com
Periodic Table Lead Pb Periodic Table Timeline Lead Pb Charge Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. 93 rows this table shows the most common charges for atoms of the chemical elements. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms. Lead Pb Charge.
From www.shutterstock.com
Lead Pb Number 82 Element Periodic Stock Vector (Royalty Free Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When a lead atom loses two electrons it exhibits a +2 charge. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. So,. Lead Pb Charge.
From www.youtube.com
Lead. Lead(pb). SCIENCE KNOWLEDGE. YouTube Lead Pb Charge Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. When a lead atom loses two electrons it exhibits a +2 charge. For example, iron(ii) has a. 93 rows this table shows the most common charges for atoms. Lead Pb Charge.
From www.shutterstock.com
Lead Pb Chemical Element Vector Illustration Stock Vector (Royalty Free Lead Pb Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. When a lead atom loses two electrons it exhibits a +2 charge. So, these two. Lead Pb Charge.
From h-o-m-e.org
An Overview on the Varying Charges of Lead (Pb) Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Sources, facts,. Lead Pb Charge.
From instrumentationtools.com
Discharge and Charging of LeadAcid Battery Inst Tools Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. 93 rows this table shows the most. Lead Pb Charge.
From material-properties.org
Lead Periodic Table and Atomic Properties Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). For example, iron(ii) has a. So, these two. Lead Pb Charge.
From www.alamy.com
Lead (Pb) symbol chemical element of the periodic table, 3D animation Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. For example, iron(ii) has a. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. When a. Lead Pb Charge.
From www.slideserve.com
PPT Lead ( Pb ) PowerPoint Presentation, free download ID1920456 Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. When a lead atom loses two electrons it exhibits a +2 charge. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead. Lead Pb Charge.
From pixels.com
Lead Pb Element Symbol Periodic Table Series 082 Mixed Media by Design Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. During bond formation, the lead atom donates two electrons from the 6p orbital to. Lead Pb Charge.
From www.perkinelmer.com
Lead (Pb) Pure Standard, 1,000 µg/mL, 2 HNO3, 500 mL PerkinElmer Lead Pb Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. 93 rows this table shows the most common. Lead Pb Charge.
From www.dreamstime.com
Lead Pb Chemical Element of Periodic Table Isolated on White Background Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a. 93 rows this table shows the most common charges for atoms of the chemical elements. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). When a lead. Lead Pb Charge.
From proper-cooking.info
Lead Atomic Model Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. For example, iron(ii) has a. Lead(iv) pb4+ roman numeral notation indicates charge of. Lead Pb Charge.
From sciencenotes.org
Lead Facts Pb or Element Number 82 Lead Pb Charge Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. 93 rows this table shows the most common charges for atoms of the chemical elements. So, these two electrons will be lost from the last shell, which means. Lead Pb Charge.
From www.alamy.com
Pb Lead Chemical Element Periodic Table. Single vector illustration Lead Pb Charge When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the. Lead Pb Charge.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Lead Pb Charge Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. 93 rows this table shows the most common charges for atoms of the chemical elements. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+).. Lead Pb Charge.
From www.shutterstock.com
Lead Symbol Pb Element Periodic Table Stock Illustration 106733897 Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. For example, iron(ii) has a. When a lead atom loses two electrons it exhibits a +2 charge. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Known in antiquity and believed. Lead Pb Charge.
From www.researchgate.net
Distribution of lead (Pb) species in aqueous medium as a function of pH Lead Pb Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). For example, iron(ii) has a. When a lead atom loses two electrons it exhibits a +2 charge. 93 rows this table shows the most common charges for atoms of the chemical elements. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.. Lead Pb Charge.
From scienceinfo.com
Lead (Pb) Element Properties, Reactions, Uses Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. For example, iron(ii) has a. During bond formation, the lead atom donates two electrons. Lead Pb Charge.
From www.researchgate.net
Mechanism of lead (Pb) toxicity Download Scientific Diagram Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When a lead. Lead Pb Charge.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(NO3)2 + KI = KNO3 + PbI2 Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is. Lead Pb Charge.
From cartoondealer.com
Pb Symbol. Lead Chemical Element RoyaltyFree Stock Photo Lead Pb Charge When a lead atom loses two electrons it exhibits a +2 charge. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. So, these two electrons will be lost from the last shell, which means electrons will be. Lead Pb Charge.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead Pb Charge When a lead atom loses two electrons it exhibits a +2 charge. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, iron(ii) has a. So, these two electrons will be lost from the last shell, which means electrons will be lost from. Lead Pb Charge.
From www.pinterest.com
Electron Configuration for Pb, Pb2+, and Pb3+ (Lead and Lead Ions Lead Pb Charge When a lead atom loses two electrons it exhibits a +2 charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a. 93 rows this table shows the most common charges for atoms of the chemical elements. So, these two. Lead Pb Charge.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When a lead atom loses two electrons it exhibits. Lead Pb Charge.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. When a lead atom loses two electrons it exhibits a +2 charge. 93 rows this table shows the most common charges for atoms of the chemical elements. During bond formation, the. Lead Pb Charge.
From www.newtondesk.com
Lead Pb Element 82 of Periodic Table Newton Desk Lead Pb Charge Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). For example, iron(ii) has a. When a lead atom loses two electrons it exhibits a +2 charge. 93 rows this table shows the. Lead Pb Charge.
From utedzz.blogspot.com
Periodic Table Lead Pb Periodic Table Timeline Lead Pb Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). For example, iron(ii) has a. When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. 93 rows. Lead Pb Charge.
From www.dreamstime.com
Pb Symbol. Lead Chemical Element Stock Illustration Illustration of Lead Pb Charge Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. Lead(iv) pb4+ roman numeral notation indicates charge of ion when element commonly forms more than one ion. So, these two. Lead Pb Charge.
From www.numerade.com
SOLVED given that the only known ionic charges of lead are pb(ii) and Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. 93 rows this table shows the most common charges for atoms of the chemical elements. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is. Lead Pb Charge.
From www.clutchprep.com
Periodic Table Charges Chemistry Video Clutch Prep Lead Pb Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When a lead atom loses two electrons it exhibits a +2 charge. For example, iron(ii) has a. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant. Lead Pb Charge.
From www.dreamstime.com
Lead Pb Stock Illustrations 42 Lead Pb Stock Illustrations, Vectors Lead Pb Charge For example, iron(ii) has a. 93 rows this table shows the most common charges for atoms of the chemical elements. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. During bond formation, the lead atom donates two electrons from the. Lead Pb Charge.
From pubs.rsc.org
Lead (Pb) interfacing with epitaxial graphene Physical Chemistry Lead Pb Charge So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. For example, iron(ii) has a. Known in antiquity and believed by the alchemists to be the oldest of metals, lead is highly durable and resistant to corrosion, as is indicated by the continuing use of lead water. Lead(iv) pb4+. Lead Pb Charge.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Pb Charge During bond formation, the lead atom donates two electrons from the 6p orbital to form a lead ion (pb 2+). When a lead atom loses two electrons it exhibits a +2 charge. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Lead Pb Charge.