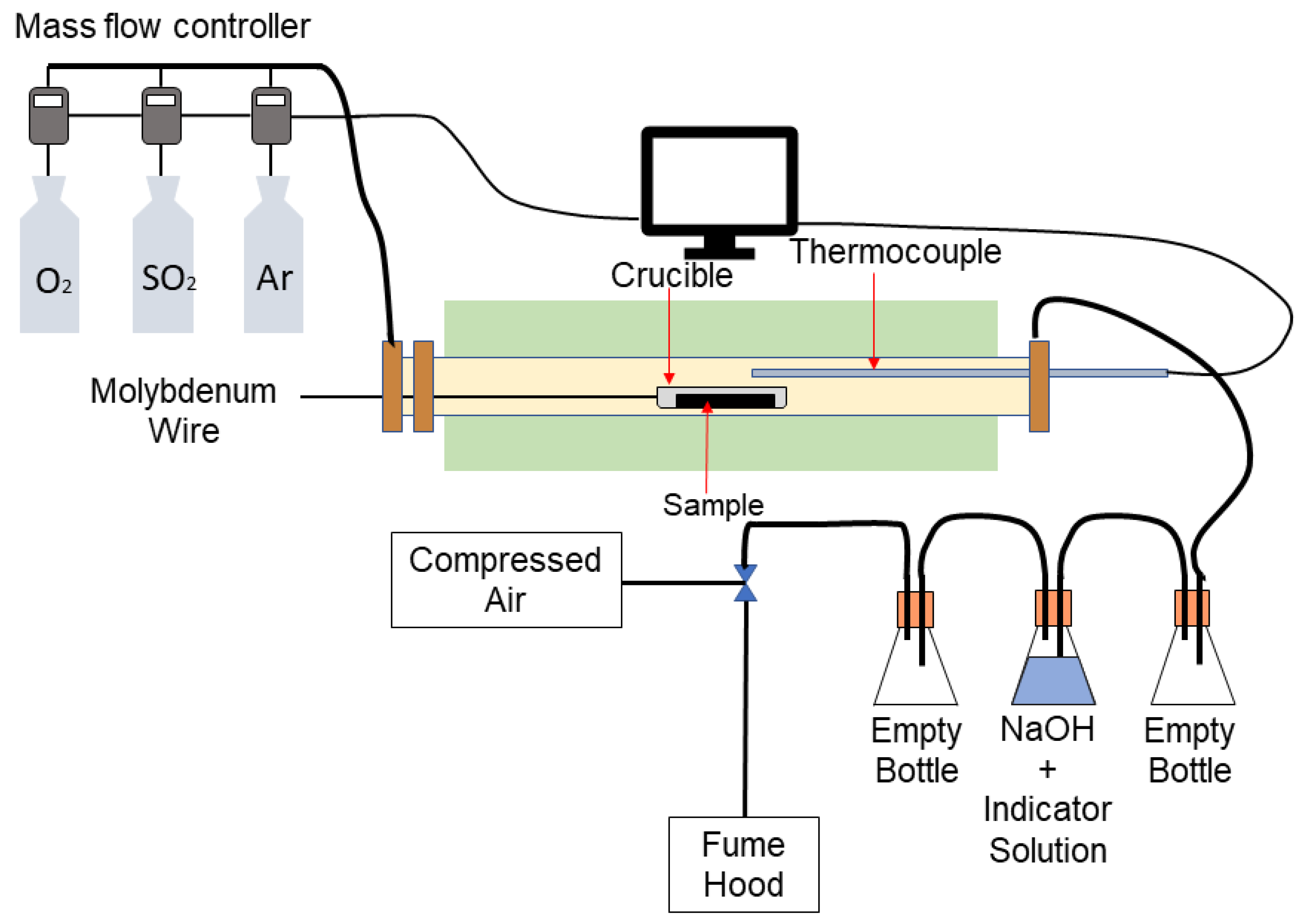
Roasting Is Used In The Extraction Of . Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. While roasting uses lower temperatures than smelting, they both use. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. The field of extractive metallurgy.
from www.mdpi.com
Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. While roasting uses lower temperatures than smelting, they both use. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide.
Metals Free FullText Selective Sulfation Roasting for Cobalt and
Roasting Is Used In The Extraction Of While roasting uses lower temperatures than smelting, they both use. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. While roasting uses lower temperatures than smelting, they both use. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form.
From www.youtube.com
Unit processes in Pyrometallurgy Drying, Roasting, Calcination Roasting Is Used In The Extraction Of Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to. Roasting Is Used In The Extraction Of.
From www.youtube.com
Class 12 Chemistry Roasting Extraction of Crude Metal Metallurgy Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. The field of. Roasting Is Used In The Extraction Of.
From www.researchgate.net
Scheme of the extraction of lead and zinc process from the oxidizing Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur. Roasting Is Used In The Extraction Of.
From ar.inspiredpencil.com
Roasting Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. While roasting uses lower temperatures than smelting, they both use. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. The field of. Roasting Is Used In The Extraction Of.
From ar.inspiredpencil.com
Coffee Roasting Process Flow Chart Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. While roasting uses lower temperatures than smelting, they both use. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Both roasting and smelting are involved in. Roasting Is Used In The Extraction Of.
From www.scottrao.com
Was it the green? The roast? The extraction? — Scott Rao Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to. Roasting Is Used In The Extraction Of.
From www.worldatlas.com
What Was The First Metal Used By Humans? WorldAtlas Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. While roasting uses lower temperatures than smelting, they both use. Roasting is the oxidation of. Roasting Is Used In The Extraction Of.
From encyclopedia.pub
Roasting Processes for Lithium Extraction Encyclopedia MDPI Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. While roasting uses lower temperatures than smelting, they. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Metals Free FullText Selective Sulfation Roasting for Cobalt and Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Despite the absence of sulphide, roasting is. Roasting Is Used In The Extraction Of.
From www.researchgate.net
Simplified flow diagram of the roastleachelectrowin (RLE) process Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Pyrometallurgy involves heating operations such as roasting,. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Minerals Free FullText Extraction of Nickel from Garnierite Roasting Is Used In The Extraction Of The field of extractive metallurgy. While roasting uses lower temperatures than smelting, they both use. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Pyrometallurgy involves heating operations such. Roasting Is Used In The Extraction Of.
From igcseandialchemistry.com
IGCSE Extraction of Metals From Ores Notes IGCSE And IAL Chemistry Roasting Is Used In The Extraction Of The field of extractive metallurgy. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. While roasting uses lower temperatures than smelting, they both use. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at. Roasting Is Used In The Extraction Of.
From www.youtube.com
Chapter 03 Topic 3 Extraction of Metals Electrolysis, Roasting Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. The field of extractive metallurgy. While roasting uses lower temperatures than smelting, they. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Materials Free FullText Effective Extraction of the Al Element Roasting Is Used In The Extraction Of Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. While roasting uses lower temperatures than smelting, they both use. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting. Roasting Is Used In The Extraction Of.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Roasting Is Used In The Extraction Of Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. While roasting uses lower temperatures than smelting, they both use. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Despite the absence of sulphide, roasting is used in the extraction of iron because (a). Roasting Is Used In The Extraction Of.
From app.pandai.org
Extraction of Metals from its Ore Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. The field of extractive metallurgy. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Pyrometallurgy involves. Roasting Is Used In The Extraction Of.
From www.semanticscholar.org
Figure 1 from Sulphation roasting process for the extraction of rare Roasting Is Used In The Extraction Of Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Despite. Roasting Is Used In The Extraction Of.
From dokumen.tips
(PDF) Sulphation roasting process for the extraction of rare DOKUMEN.TIPS Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. While roasting uses lower temperatures than smelting, they both use. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal.. Roasting Is Used In The Extraction Of.
From www.tuttee.co
CHEM Extraction of Metal chemistry metal extraction blast furnace Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about. Roasting Is Used In The Extraction Of.
From www.teachoo.com
Extraction of Metals How is it carried out? Different processes exp Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. While roasting uses lower temperatures than smelting,. Roasting Is Used In The Extraction Of.
From www.youtube.com
Metallurgy, Extraction of metal, roasting, smelting and bessmerisation Roasting Is Used In The Extraction Of Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. While roasting uses lower. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Processes Free FullText Pilot Study on a New Conveyor Bed Roasting Is Used In The Extraction Of Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. The field of extractive metallurgy. Extractive metallurgy is the practice. Roasting Is Used In The Extraction Of.
From www.w3schools.blog
Iron Extraction W3schools Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. The field of extractive metallurgy. While roasting uses lower temperatures than smelting, they. Roasting Is Used In The Extraction Of.
From dailycoffeenews.com
How Might Roasting Affect Drip Extraction A Detailed Analysis Daily Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. The field of extractive metallurgy. While roasting uses lower temperatures than smelting, they both use. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting is a metallurgical process that involves the heating of metal ores or concentrates. Roasting Is Used In The Extraction Of.
From www.researchgate.net
Effects of roasting temperature (a) and roasting time (b) on Ni Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Extractive metallurgy is the practice. Roasting Is Used In The Extraction Of.
From justinbox.weebly.com
Heat and Mass Transfer JUSTIN BOX Roasting Is Used In The Extraction Of The field of extractive metallurgy. While roasting uses lower temperatures than smelting, they both use. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting is a metallurgical process that involves. Roasting Is Used In The Extraction Of.
From www.youtube.com
Roasting and Calcination of Ore (Extraction of crude metal)IITJEE,NEET Roasting Is Used In The Extraction Of While roasting uses lower temperatures than smelting, they both use. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Pyrometallurgy involves heating operations such as roasting, in which compounds are. Roasting Is Used In The Extraction Of.
From www.researchgate.net
Effect of roasting time on copper extraction (500˚C, 1 Na 2 SO 3 Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. While roasting uses lower temperatures than smelting, they both use. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Extractive metallurgy is the practice of removing valuable metals from. Roasting Is Used In The Extraction Of.
From www.youtube.com
Roasting,Calcination & Extraction Of ZincPart 12cbsegrade12 Roasting Is Used In The Extraction Of Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Despite. Roasting Is Used In The Extraction Of.
From ijmmm.ustb.edu.cn
Fluidized roasting of refractory sideritecontaining iron Roasting Is Used In The Extraction Of Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted. Roasting Is Used In The Extraction Of.
From www.researchgate.net
Effect of roasting time on extraction of Ca, Mg, and Al (roasting Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Both roasting and smelting are involved in preparing the ore for the final extraction of the desired metal. The field of extractive metallurgy. Despite the absence of sulphide, roasting. Roasting Is Used In The Extraction Of.
From www.thespruceeats.com
Barding A Medieval Roasting Technique Worth Pondering Roasting Is Used In The Extraction Of Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed (b) all. Both roasting and smelting are involved in preparing the ore. Roasting Is Used In The Extraction Of.
From www.youtube.com
Roasting and calcination extraction of metal Chemistry Khan Roasting Is Used In The Extraction Of The field of extractive metallurgy. Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. Both roasting and. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Metals Free FullText Selective Sulfation Roasting for Cobalt and Roasting Is Used In The Extraction Of Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into purer form. The field of extractive metallurgy. Roasting is a metallurgical process that involves the heating of metal ores or concentrates in the presence of air to bring about a thermal. While roasting uses lower temperatures than smelting, they both use.. Roasting Is Used In The Extraction Of.
From www.mdpi.com
Processes Free FullText Mechanism and of Ammonium Sulfate Roasting Is Used In The Extraction Of Roasting is the oxidation of metal sulphides to give metal oxides and sulphur dioxide. The field of extractive metallurgy. Pyrometallurgy involves heating operations such as roasting, in which compounds are converted at temperatures just below their. Despite the absence of sulphide, roasting is used in the extraction of iron because (a) fe 2 o 3 (hematite) is to be decomposed. Roasting Is Used In The Extraction Of.