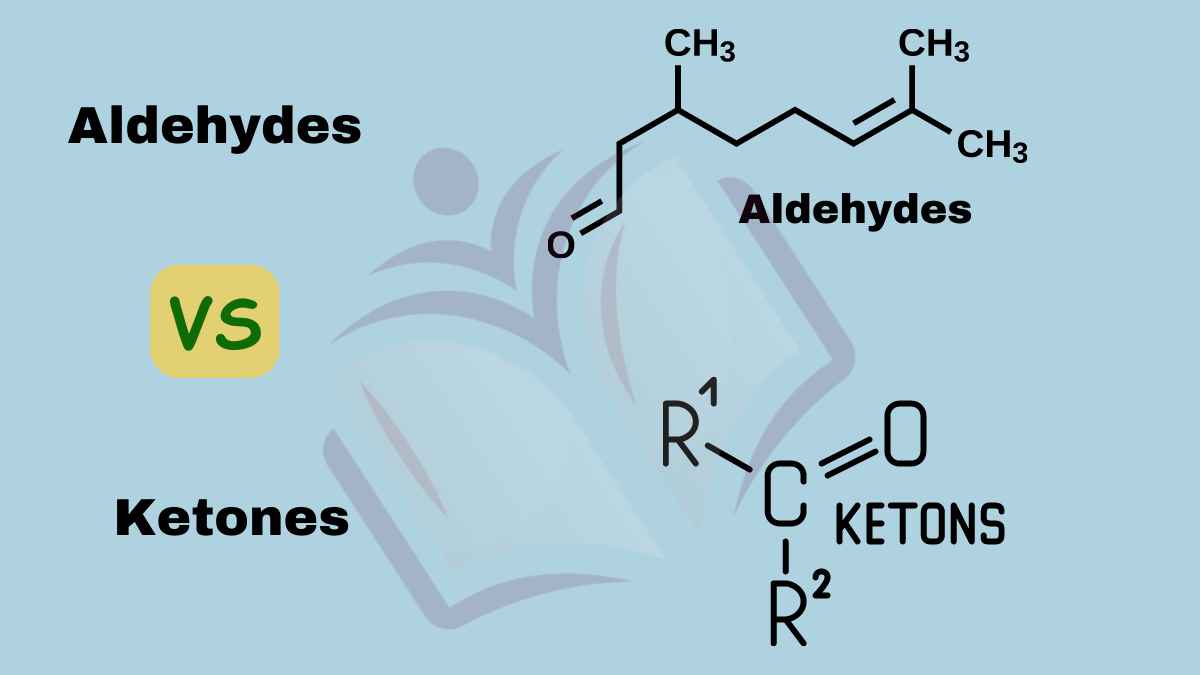
Aldehyde Versus Ketone . It also considers their simple physical. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. In a ketone, the carbonyl group is bonded to two carbon atoms: Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. The carbon atom of this group has two remaining. They differ greatly, however, in one most important type of reaction: This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Here are five key differences between them: In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom.
from differguide.com
Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. They differ greatly, however, in one most important type of reaction: Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Here are five key differences between them: The carbon atom of this group has two remaining. In a ketone, the carbonyl group is bonded to two carbon atoms: It also considers their simple physical. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation.
Difference Between Aldehydes And Ketones
Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. The carbon atom of this group has two remaining. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. It also considers their simple physical. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. They differ greatly, however, in one most important type of reaction: Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Here are five key differences between them: In a ketone, the carbonyl group is bonded to two carbon atoms: Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation.
From www.masterorganicchemistry.com
The Simple TwoStep Pattern For Seven Key Reactions of Aldehydes and Aldehyde Versus Ketone Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Here are five key differences between them:. Aldehyde Versus Ketone.
From www.slideserve.com
PPT Aldehydes and Ketones PowerPoint Presentation, free download ID Aldehyde Versus Ketone This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Here are five key differences between them: The carbon atom of this group has two remaining. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. It also. Aldehyde Versus Ketone.
From www.youtube.com
Aldehyde, Ketone and Carboxylic Acid Structure, Properties and Aldehyde Versus Ketone The carbon atom of this group has two remaining. It also considers their simple physical. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. They differ greatly, however, in one most important type of reaction: In a ketone, the carbonyl group is bonded to two carbon atoms: Ketone is a class of organic compounds that. Aldehyde Versus Ketone.
From www.masterorganicchemistry.com
The Simple TwoStep Pattern For Seven Key Reactions of Aldehydes and Aldehyde Versus Ketone The carbon atom of this group has two remaining. In a ketone, the carbonyl group is bonded to two carbon atoms: Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. It also considers their simple physical. In an aldehyde, the carbonyl group is bonded to at least. Aldehyde Versus Ketone.
From collegedunia.com
Difference Between Aldehydes and Ketones Aldehyde Versus Ketone Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. The carbon atom of this group has two remaining. In a ketone, the carbonyl group is bonded to two carbon. Aldehyde Versus Ketone.
From chem.libretexts.org
Natural Occurrence of Aldehydes and Ketones Chemistry LibreTexts Aldehyde Versus Ketone In a ketone, the carbonyl group is bonded to two carbon atoms: This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. They differ greatly, however, in one most important. Aldehyde Versus Ketone.
From www.slideserve.com
PPT Aldehyde and ketones PowerPoint Presentation, free download ID Aldehyde Versus Ketone Here are five key differences between them: They differ greatly, however, in one most important type of reaction: Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. The carbon atom of this group has. Aldehyde Versus Ketone.
From srjng88.medium.com
What is the difference between aldehydes and ketones? by Chemistry Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. The carbon atom of this group has two remaining. Here are five key differences between them: This page explains what aldehydes and ketones are, and. Aldehyde Versus Ketone.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Aldehyde Versus Ketone In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Ketone is a class of organic compounds that are characterized by the presence of a. Aldehyde Versus Ketone.
From www.youtube.com
The difference between aldehydes and ketones YouTube Aldehyde Versus Ketone They differ greatly, however, in one most important type of reaction: In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. It also. Aldehyde Versus Ketone.
From www.slideserve.com
PPT ALDEHYDES AND KETONES PowerPoint Presentation, free download ID Aldehyde Versus Ketone In a ketone, the carbonyl group is bonded to two carbon atoms: Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. In an aldehyde, the. Aldehyde Versus Ketone.
From www.slideserve.com
PPT ALDEHYDES AND KETONES PowerPoint Presentation, free download ID Aldehyde Versus Ketone This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Here are five key differences between them: In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. It also considers their simple physical. They differ greatly, however,. Aldehyde Versus Ketone.
From saylordotorg.github.io
Aldehydes and Ketones Structure and Names Aldehyde Versus Ketone In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Here are five key differences between them: In a ketone, the carbonyl group is bonded to two carbon atoms: Aldehydes and ketones are much alike in many of their reactions, owing to the. Aldehyde Versus Ketone.
From differguide.com
Difference Between Aldehydes And Ketones Aldehyde Versus Ketone It also considers their simple physical. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Here are five key differences between them: In a ketone,. Aldehyde Versus Ketone.
From chem.libretexts.org
19.S Aldehydes and Ketones (Summary) Chemistry LibreTexts Aldehyde Versus Ketone Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. This page explains what aldehydes and ketones are, and looks at the way. Aldehyde Versus Ketone.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation ID233852 Aldehyde Versus Ketone Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. They differ greatly, however, in one most important type of reaction: Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. In a ketone, the carbonyl group is bonded to two carbon atoms: It also. Aldehyde Versus Ketone.
From brainkart.com
Chemical properties of aldehydes and ketones Aldehyde Versus Ketone In a ketone, the carbonyl group is bonded to two carbon atoms: The carbon atom of this group has two remaining. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. This page explains what aldehydes and. Aldehyde Versus Ketone.
From www.studocu.com
Aldehyde and Ketone Structure, Preparation, Properties, Uses Aldehyde Versus Ketone This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. The carbon atom of this group has two remaining. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group.. Aldehyde Versus Ketone.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Aldehydes & Ketones Aldehyde Versus Ketone They differ greatly, however, in one most important type of reaction: Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o.. Aldehyde Versus Ketone.
From pediaa.com
Difference Between Aldehyde and Ketone Structure, Properties, Naming Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes and ketones are much alike in many of their reactions,. Aldehyde Versus Ketone.
From chem.libretexts.org
12.1 The Nomenclature of Aldehydes and Ketones Chemistry LibreTexts Aldehyde Versus Ketone Here are five key differences between them: Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. In a ketone, the carbonyl group is bonded to two carbon atoms: It also considers their simple physical. The carbon atom of this group has two remaining. This page. Aldehyde Versus Ketone.
From www.youtube.com
Hydration of aldehydes and Ketones YouTube Aldehyde Versus Ketone Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. In a ketone, the carbonyl group is bonded to two carbon atoms: This page explains what aldehydes and ketones are, and looks at the way. Aldehyde Versus Ketone.
From www.youtube.com
Aldehydes and Ketones YouTube Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. It also considers their simple physical. They differ greatly, however, in. Aldehyde Versus Ketone.
From www.chemistrysteps.com
Reactions of Aldehydes and Ketones with Water Chemistry Steps Aldehyde Versus Ketone The carbon atom of this group has two remaining. It also considers their simple physical. Here are five key differences between them: Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. This page explains what aldehydes and ketones are, and looks at the way their bonding affects. Aldehyde Versus Ketone.
From www.pinterest.com
Difference between Aldehyde and Ketone Ketones, Chemistry notes Aldehyde Versus Ketone Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. The carbon atom of this group has two remaining. Here are five key differences between them: In a ketone, the carbonyl group is bonded to two carbon atoms: It also considers their simple physical. Aldehydes and ketones are much alike in many of their reactions, owing to the presence. Aldehyde Versus Ketone.
From www.diffzy.com
Aldehydes vs. Ketones What's The Difference (With Table) Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. In a ketone, the carbonyl group is bonded to two carbon atoms: Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. The carbon. Aldehyde Versus Ketone.
From chem.libretexts.org
12.1 The Nomenclature of Aldehydes and Ketones Chemistry LibreTexts Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Here are five key differences between them: In a ketone, the carbonyl group is bonded to two carbon atoms: Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. This page explains what aldehydes and. Aldehyde Versus Ketone.
From chem.libretexts.org
12.1 The Nomenclature of Aldehydes and Ketones Chemistry LibreTexts Aldehyde Versus Ketone Here are five key differences between them: In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. It also considers their simple physical.. Aldehyde Versus Ketone.
From chem.libretexts.org
12.1 The Nomenclature of Aldehydes and Ketones Chemistry LibreTexts Aldehyde Versus Ketone This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Here are five key differences between them: Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. It also considers their simple physical. The carbon atom of this group has. Aldehyde Versus Ketone.
From www.pdfprof.com
aldehydes ketones and carboxylic acids neet notes pdf Aldehyde Versus Ketone Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. In an aldehyde, the carbonyl group is bonded to. Aldehyde Versus Ketone.
From www.tes.com
Aldehydes and Ketones OCR A Level Teaching Resources Aldehyde Versus Ketone Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. It also considers their simple physical. In a ketone, the carbonyl group is bonded to two carbon atoms: They differ greatly, however, in one most important type. Aldehyde Versus Ketone.
From pediaa.com
Difference Between Aldehyde and Ketone Structure, Properties, Naming Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Here are five key differences between them:. Aldehyde Versus Ketone.
From www.doccheck.com
Aldehyd und Keton DocCheck Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. This page explains what aldehydes and ketones are, and looks at the way their bonding affects their reactivity. It also considers their simple physical. The. Aldehyde Versus Ketone.
From chem.libretexts.org
19.S Aldehydes and Ketones (Summary) Chemistry LibreTexts Aldehyde Versus Ketone Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. Ketone is a class of organic compounds that are characterized by the presence of a carbonyl group (co) as a functional group. This page explains what aldehydes and ketones are, and looks at the way their bonding affects. Aldehyde Versus Ketone.
From www.organicchemistrytutor.com
Reactions of Aldehydes and Ketones — Organic Chemistry Tutor Aldehyde Versus Ketone Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, c=o. The carbon atom of this group has two remaining. Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. In a ketone, the carbonyl group is bonded to two carbon atoms: Ketone is a class. Aldehyde Versus Ketone.