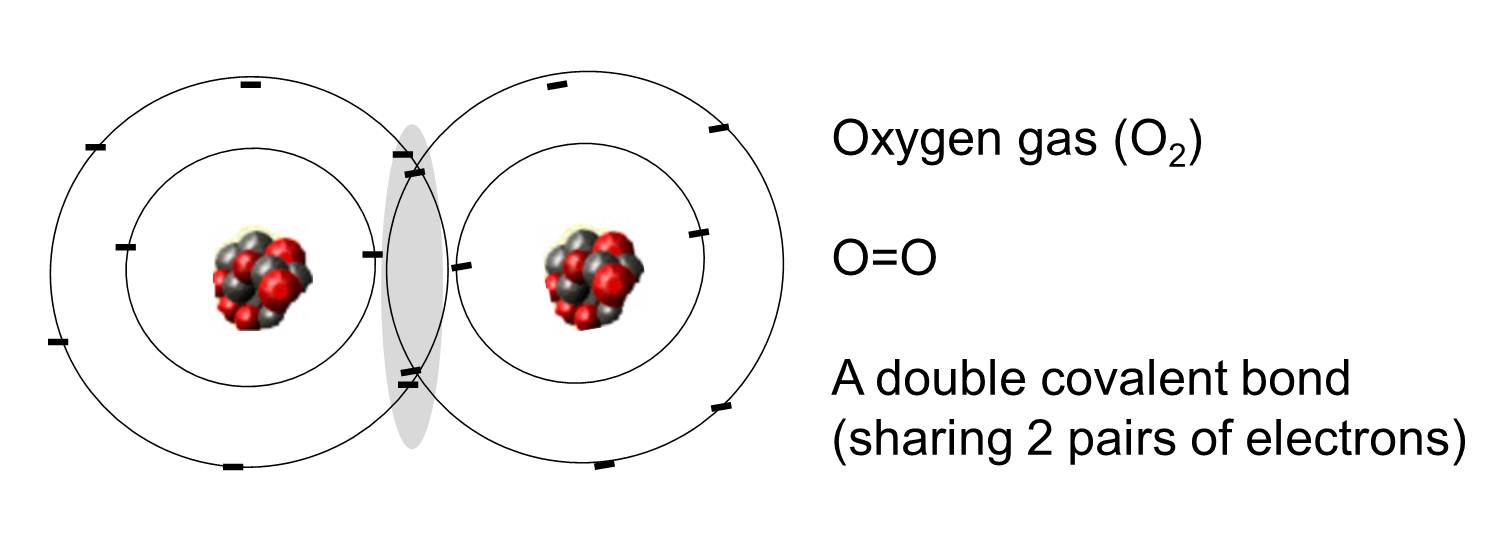
What Are Electrons Shared In Molecular Compounds . It is this sharing of electrons that we refer to as a. each atom donates a single electron to the electron pair which is shared. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The electrons involved are in the outer shells of the atoms. Let us illustrate a covalent bond by. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. when electrons are shared between two atoms, they make a bond called a covalent bond. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. An atom that shares one or. share a pair of.
from sphweb.bumc.bu.edu
a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. An atom that shares one or. Let us illustrate a covalent bond by. The electrons involved are in the outer shells of the atoms. each atom donates a single electron to the electron pair which is shared. It is this sharing of electrons that we refer to as a. when electrons are shared between two atoms, they make a bond called a covalent bond. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. share a pair of. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms.
Basic Cell Biology
What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. It is this sharing of electrons that we refer to as a. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The electrons involved are in the outer shells of the atoms. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. when electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or. each atom donates a single electron to the electron pair which is shared. share a pair of. Let us illustrate a covalent bond by.
From courses.lumenlearning.com
The Building Blocks of Molecules Biology I What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. covalent bond,. What Are Electrons Shared In Molecular Compounds.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Are Electrons Shared In Molecular Compounds a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. share a pair of. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares one or. It is this sharing of electrons that we. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Let us illustrate a covalent bond by. each atom donates a single electron to the electron pair which is shared. The electrons. What Are Electrons Shared In Molecular Compounds.
From www.chemistrylearner.com
Lewis Dot Structure Definition, Examples, and Drawing What Are Electrons Shared In Molecular Compounds The electrons involved are in the outer shells of the atoms. share a pair of. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. An atom that shares one or. It is this sharing of electrons that we refer to as a.. What Are Electrons Shared In Molecular Compounds.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Are Electrons Shared In Molecular Compounds a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. It is this sharing of electrons that we refer to as a. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. An atom. What Are Electrons Shared In Molecular Compounds.
From www.tec-science.com
Covalent bonding tecscience What Are Electrons Shared In Molecular Compounds share a pair of. when electrons are shared between two atoms, they make a bond called a covalent bond. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. It is this sharing of electrons that we refer to as a. Let. What Are Electrons Shared In Molecular Compounds.
From saylordotorg.github.io
Molecular Geometry and Covalent Bonding Models What Are Electrons Shared In Molecular Compounds a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. share a pair of. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The electrons involved are in the outer shells of. What Are Electrons Shared In Molecular Compounds.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Are Electrons Shared In Molecular Compounds each atom donates a single electron to the electron pair which is shared. An atom that shares one or. The electrons involved are in the outer shells of the atoms. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. share a. What Are Electrons Shared In Molecular Compounds.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. Let us illustrate a covalent bond by. each atom donates a single electron to the electron pair which is shared. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a species formed from covalently. What Are Electrons Shared In Molecular Compounds.
From slidetodoc.com
Covalent Compounds Lewis Dot Structure polarity molecule shapes What Are Electrons Shared In Molecular Compounds covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. share a pair of. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Let us illustrate a covalent bond by. a covalent bond is a chemical. What Are Electrons Shared In Molecular Compounds.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. share a pair of. An atom that shares one or. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a species formed from covalently bonded atoms is a molecule and is represented by a. What Are Electrons Shared In Molecular Compounds.
From courses.lumenlearning.com
Molecular Structure and Polarity Chemistry What Are Electrons Shared In Molecular Compounds each atom donates a single electron to the electron pair which is shared. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. share a pair of. Let us illustrate a covalent bond by. An atom that shares one or. when electrons are shared between two atoms,. What Are Electrons Shared In Molecular Compounds.
From www.expii.com
Ionic Bond — Formation & Compounds Expii What Are Electrons Shared In Molecular Compounds the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Let us illustrate a covalent bond by. share a pair of. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. . What Are Electrons Shared In Molecular Compounds.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Are Electrons Shared In Molecular Compounds the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the outer shells of the atoms. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. covalent. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Are Electrons Shared In Molecular Compounds covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Let us illustrate a covalent bond by. The electrons involved are in the outer shells of the atoms. It is this sharing of electrons that we refer to as a. when electrons are shared between two atoms, they make. What Are Electrons Shared In Molecular Compounds.
From www.wou.edu
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry What Are Electrons Shared In Molecular Compounds The electrons involved are in the outer shells of the atoms. when electrons are shared between two atoms, they make a bond called a covalent bond. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. An atom that shares one or. It is this sharing of electrons that. What Are Electrons Shared In Molecular Compounds.
From sciencenotes.org
How to Draw a Lewis Structure What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The electrons involved are in the outer shells of the atoms. Let us illustrate a covalent bond by. each atom donates a single electron to the electron. What Are Electrons Shared In Molecular Compounds.
From www.pinterest.com
Four covalent bonds. Carbon has four valence electrons and here a What Are Electrons Shared In Molecular Compounds share a pair of. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares one or. It is. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Review of Ionic Compounds PowerPoint Presentation, free download What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. The electrons involved are in the outer shells of the atoms. when electrons are shared between two atoms, they make a bond called a covalent bond. each atom donates a single electron to the electron pair which is shared. share a pair of. a. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Section 1 Atoms, Bonding, and the Periodic Table PowerPoint What Are Electrons Shared In Molecular Compounds share a pair of. Let us illustrate a covalent bond by. The electrons involved are in the outer shells of the atoms. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. when electrons are shared between two atoms, they make a bond called a covalent bond. An. What Are Electrons Shared In Molecular Compounds.
From saylordotorg.github.io
Lewis Structures and Covalent Bonding What Are Electrons Shared In Molecular Compounds a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. It is this sharing of electrons that we refer to as a. The electrons involved are in the outer shells of the atoms. Let us illustrate a covalent bond by. An atom that shares one or. share a pair. What Are Electrons Shared In Molecular Compounds.
From www.expii.com
Multiple Bonds — Double & Triple Bonds Expii What Are Electrons Shared In Molecular Compounds Let us illustrate a covalent bond by. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the outer shells of the atoms. when electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. each atom. What Are Electrons Shared In Molecular Compounds.
From byjus.com
The total number of electrons shared between the atoms of a hydrogen What Are Electrons Shared In Molecular Compounds share a pair of. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. when electrons are shared between two atoms, they make a bond called a. What Are Electrons Shared In Molecular Compounds.
From chemdictionary.org
Double Covalent Bond Facts, Definition, History & Examples What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Let us illustrate a covalent bond by. The electrons involved are in the outer shells of the atoms. each atom donates a single electron to the electron. What Are Electrons Shared In Molecular Compounds.
From www.slideserve.com
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint What Are Electrons Shared In Molecular Compounds a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Let us illustrate a covalent bond by. share a pair of. An atom that shares one or. when electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons. What Are Electrons Shared In Molecular Compounds.
From biologydictionary.net
Covalent Bond Biology Dictionary What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. share a pair of. each atom donates a single electron to the electron pair which is shared. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. a species formed. What Are Electrons Shared In Molecular Compounds.
From slideplayer.com
Covalent Bonding. ppt download What Are Electrons Shared In Molecular Compounds Let us illustrate a covalent bond by. An atom that shares one or. each atom donates a single electron to the electron pair which is shared. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The electrons involved are in the outer shells of the atoms. the. What Are Electrons Shared In Molecular Compounds.
From sphweb.bumc.bu.edu
Basic Cell Biology What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by. share a pair of. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. a species formed from covalently bonded atoms is a molecule and. What Are Electrons Shared In Molecular Compounds.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Are Electrons Shared In Molecular Compounds when electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. It is this sharing of electrons that we refer to as a. . What Are Electrons Shared In Molecular Compounds.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table What Are Electrons Shared In Molecular Compounds Let us illustrate a covalent bond by. It is this sharing of electrons that we refer to as a. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. when electrons are shared between two atoms, they make a bond called a covalent. What Are Electrons Shared In Molecular Compounds.
From emedia.rmit.edu.au
Covalent bonds Learning Lab What Are Electrons Shared In Molecular Compounds Let us illustrate a covalent bond by. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. each atom donates a single electron to the electron pair which. What Are Electrons Shared In Molecular Compounds.
From heuristic-ritchie-0d4648.netlify.app
Electron Configuration Of Oxygen In Ground State What Are Electrons Shared In Molecular Compounds Let us illustrate a covalent bond by. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. It is this sharing of electrons that we refer to as a. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond.. What Are Electrons Shared In Molecular Compounds.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Are Electrons Shared In Molecular Compounds share a pair of. a species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each. when electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a. An. What Are Electrons Shared In Molecular Compounds.
From chem.libretexts.org
2.6 Molecular and Ionic Compounds Chemistry LibreTexts What Are Electrons Shared In Molecular Compounds It is this sharing of electrons that we refer to as a. the sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. when electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms.. What Are Electrons Shared In Molecular Compounds.