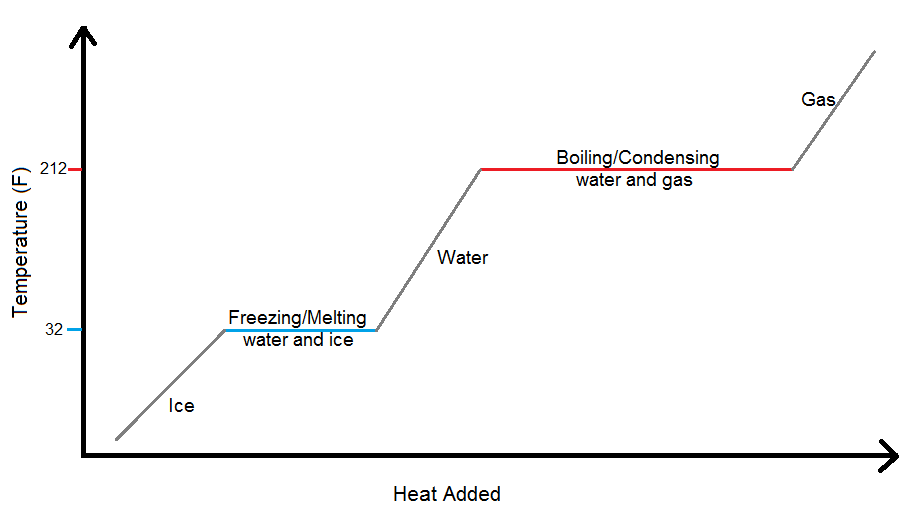
What Temperature Changes Occur When Ice Changes To Water . water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. As the solid ice is heated, the temperature. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the.
from socratic.org
this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. In region 4 our mass exists in two state, water and. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. As the solid ice is heated, the temperature.
How would you use the phase diagram of water to explain why ice at the
What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. In region 4 our mass exists in two state, water and. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. As the solid ice is heated, the temperature.
From www.exploratorium.edu
Global Climate Change Explorer Ice, Glaciers, and the Poles What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. . What Temperature Changes Occur When Ice Changes To Water.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC What Temperature Changes Occur When Ice Changes To Water the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is. What Temperature Changes Occur When Ice Changes To Water.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. water has the unusual property that. What Temperature Changes Occur When Ice Changes To Water.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. this plot of temperature shows. What Temperature Changes Occur When Ice Changes To Water.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into. What Temperature Changes Occur When Ice Changes To Water.
From www.gauthmath.com
The plot below, called a phase diagram, shows several phases of water What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual. What Temperature Changes Occur When Ice Changes To Water.
From socratic.org
What happens to the energy of its molecules as ice melts into What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. In region 4 our mass exists in two state, water and. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight. What Temperature Changes Occur When Ice Changes To Water.
From leverageedu.com
What Are Physical and Chemical Changes Leverage Edu What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. . What Temperature Changes Occur When Ice Changes To Water.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. this plot of. What Temperature Changes Occur When Ice Changes To Water.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature,. What Temperature Changes Occur When Ice Changes To Water.
From www.shutterstock.com
Temperature Ice And Boiling Water Stock Vector 303198758 Shutterstock What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. In region 4 our mass exists in two state, water and. this plot of. What Temperature Changes Occur When Ice Changes To Water.
From www.slideserve.com
PPT Properties of Matter PowerPoint Presentation, free download ID What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially. What Temperature Changes Occur When Ice Changes To Water.
From www.researchgate.net
Pressuretemperature phase diagram of water liquid phase and solid ice What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. . What Temperature Changes Occur When Ice Changes To Water.
From easyscienceforkids.com
Changes in Matter Phases States of Matter Image What Temperature Changes Occur When Ice Changes To Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. In region 4 our mass exists in two state, water and. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. when. What Temperature Changes Occur When Ice Changes To Water.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation What Temperature Changes Occur When Ice Changes To Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. In region 4 our mass exists in two state, water and. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is. What Temperature Changes Occur When Ice Changes To Water.
From stock.adobe.com
Change of State Water. State of matter. Change of water according to What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature,. What Temperature Changes Occur When Ice Changes To Water.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Ice's Thermal What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c. What Temperature Changes Occur When Ice Changes To Water.
From brainly.in
To study the effect of heat on ice by using a graph Brainly.in What Temperature Changes Occur When Ice Changes To Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample. What Temperature Changes Occur When Ice Changes To Water.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense. What Temperature Changes Occur When Ice Changes To Water.
From wt.kimiq.com
Heating Curve Of Water Water Ionizer What Temperature Changes Occur When Ice Changes To Water the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. As the solid ice is heated, the temperature. . What Temperature Changes Occur When Ice Changes To Water.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. As the solid ice is heated, the temperature. In region 4 our mass exists in two state, water and. the constancy of temperature when water is going through its phase. What Temperature Changes Occur When Ice Changes To Water.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. the constancy of temperature when water is going through its phase changes at 0°c. What Temperature Changes Occur When Ice Changes To Water.
From francescopochetti.com
ice Archives What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense than liquid water at the melting point, so at. What Temperature Changes Occur When Ice Changes To Water.
From www3.epa.gov
Climate Impacts on Water Resources Climate Change US EPA What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its phase changes at. What Temperature Changes Occur When Ice Changes To Water.
From socratic.org
How would you use the phase diagram of water to explain why ice at the What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1. What Temperature Changes Occur When Ice Changes To Water.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Temperature Changes Occur When Ice Changes To Water the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. this plot of temperature shows what happens to a 75 g. What Temperature Changes Occur When Ice Changes To Water.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. As the solid ice. What Temperature Changes Occur When Ice Changes To Water.
From www.youtube.com
what is the final temperature when ice is added to water? YouTube What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. water has the unusual property that ice is less dense. What Temperature Changes Occur When Ice Changes To Water.
From www.kidpid.com
States of Water Kidpid What Temperature Changes Occur When Ice Changes To Water As the solid ice is heated, the temperature. water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature. What Temperature Changes Occur When Ice Changes To Water.
From socratic.org
What is the profile of the graph of temperature versus time, when water What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. the constancy of temperature when water is going through its. What Temperature Changes Occur When Ice Changes To Water.
From www.snexplores.org
Explainer What are the different states of matter? What Temperature Changes Occur When Ice Changes To Water In region 4 our mass exists in two state, water and. As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. when the temperature of the water becomes 100 ºc, it starts. What Temperature Changes Occur When Ice Changes To Water.
From www.esa.int
ESA Phase transitions between ice, water and vapour, and the energy What Temperature Changes Occur When Ice Changes To Water when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. In region 4 our mass exists in two state, water and. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:.. What Temperature Changes Occur When Ice Changes To Water.
From www.pinterest.com
Water Expansion When Freezing Science Facts Water molecule What Temperature Changes Occur When Ice Changes To Water the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. As the solid ice is heated, the temperature. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. In region 4 our. What Temperature Changes Occur When Ice Changes To Water.
From www.quora.com
What makes water, ice, and steam different from one another? Quora What Temperature Changes Occur When Ice Changes To Water water has the unusual property that ice is less dense than liquid water at the melting point, so at a fixed temperature, you can. In region 4 our mass exists in two state, water and. when the temperature of the water becomes 100 ºc, it starts to boil and evaporation of it speeds up. As the solid ice. What Temperature Changes Occur When Ice Changes To Water.
From hrsbstaff.ednet.ns.ca
Science 10 What Temperature Changes Occur When Ice Changes To Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. the constancy of temperature when water is going through its phase changes at 0°c and 100°c provides some insight into the. this plot of temperature shows what happens to a 75 g. What Temperature Changes Occur When Ice Changes To Water.