What Bond Is Electrons Are Shared Unequally . It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. Covalent bonds result from the sharing of electrons between atoms. Chemists frequently use lewis diagrams to. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond in which. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A covalent bond in which the electrons are shared equally by the two atoms. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. There are two types of covalent bonding:
from www.nagwa.com
When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond in which. Covalent bonds result from the sharing of electrons between atoms. There are two types of covalent bonding: A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Chemists frequently use lewis diagrams to. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A covalent bond in which the electrons are shared equally by the two atoms.
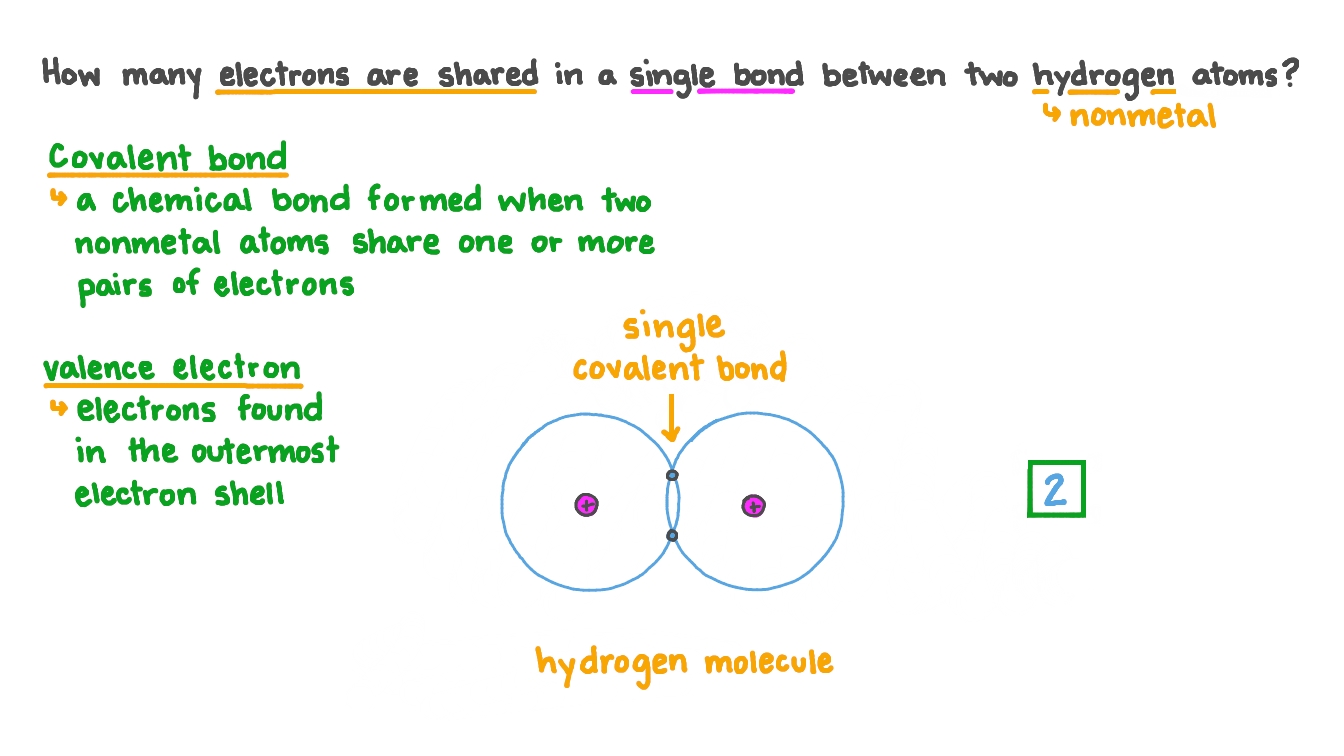
Question Video Determining How Many Electrons Are Shared in a Single
What Bond Is Electrons Are Shared Unequally It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. When electrons are shared between two atoms, they make a bond called a covalent bond. Covalent bonds result from the sharing of electrons between atoms. Chemists frequently use lewis diagrams to. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. There are two types of covalent bonding: Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A covalent bond in which the electrons are shared equally by the two atoms. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. A covalent bond in which.
From slideplayer.com
Section 4 Covalent bonding ppt download What Bond Is Electrons Are Shared Unequally There are two types of covalent bonding: Covalent bonds result from the sharing of electrons between atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to.. What Bond Is Electrons Are Shared Unequally.
From www.science-revision.co.uk
Polar bonds and molecules What Bond Is Electrons Are Shared Unequally A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. When electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Chemical Bonds & Reactions ppt download What Bond Is Electrons Are Shared Unequally Covalent bonds result from the sharing of electrons between atoms. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. A covalent bond in which the electrons are shared equally by the two atoms. A covalent bond in which. It is this sharing of electrons that we. What Bond Is Electrons Are Shared Unequally.
From www.numerade.com
SOLVED bond where the electrons are shared unequally is called a(an What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. A covalent bond in which. There are two types of covalent bonding: Covalent bonds result from the sharing. What Bond Is Electrons Are Shared Unequally.
From www.numerade.com
SOLVEDWhat happens to electrons in the formation of a polar covalent What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named. What Bond Is Electrons Are Shared Unequally.
From slidetodoc.com
Chemical Bonds Three basic types Ionic Electrostatic attraction What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. Chemists frequently use lewis diagrams to. Covalent bonds result from the sharing of electrons between atoms. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. A covalent bond in which. It is. What Bond Is Electrons Are Shared Unequally.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I What Bond Is Electrons Are Shared Unequally A covalent bond in which. There are two types of covalent bonding: When electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. A covalent bond in which. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Bonding Chapters ppt download What Bond Is Electrons Are Shared Unequally A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. Chemists frequently use lewis diagrams to. There are two types of covalent bonding: Covalent bonds result. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
When a chemical bond is broken, energy is ppt download What Bond Is Electrons Are Shared Unequally Covalent bonds result from the sharing of electrons between atoms. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. There are two types of covalent bonding: It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID909034 What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. There are two types of covalent bonding: A covalent bond in which. Chemists frequently use lewis diagrams to. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. It is this sharing of. What Bond Is Electrons Are Shared Unequally.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Bond Is Electrons Are Shared Unequally A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A covalent bond in which the electrons are shared equally by the two atoms. A covalent. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Covalent Bonding Covalent bond Bonds between two nonmetals. Electrons What Bond Is Electrons Are Shared Unequally Chemists frequently use lewis diagrams to. Covalent bonds result from the sharing of electrons between atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond in which. There are two types of covalent bonding: A covalent bond in which the electrons are shared equally by the two atoms. A molecule that. What Bond Is Electrons Are Shared Unequally.
From surfguppy.com
bonding Archives Surfguppy Chemistry made easy visual learning What Bond Is Electrons Are Shared Unequally A covalent bond in which. A covalent bond in which the electrons are shared equally by the two atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. There are two types of covalent bonding: It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free What Bond Is Electrons Are Shared Unequally Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. Covalent bonds result from the sharing of electrons between atoms. Chemists frequently use lewis diagrams to. A covalent bond in which the electrons are shared equally by the two atoms. When electrons are shared between two atoms, they make. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Chapter 12 Covalent Bonding ppt download What Bond Is Electrons Are Shared Unequally When electrons are shared between two atoms, they make a bond called a covalent bond. Covalent bonds result from the sharing of electrons between atoms. There are two types of covalent bonding: A covalent bond in which. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. It is. What Bond Is Electrons Are Shared Unequally.
From askfilo.com
Triple covalent bond When 3 pairs of electrons are shared. forey (1).. What Bond Is Electrons Are Shared Unequally A covalent bond in which. When electrons are shared between two atoms, they make a bond called a covalent bond. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. There are two types of covalent bonding: It is this sharing of electrons that we refer to as a. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Section 1 Atoms, Bonding, and the Periodic Table PowerPoint What Bond Is Electrons Are Shared Unequally A covalent bond in which. There are two types of covalent bonding: Chemists frequently use lewis diagrams to. A covalent bond in which the electrons are shared equally by the two atoms. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. It is this sharing of electrons that. What Bond Is Electrons Are Shared Unequally.
From higheducationlearning.com
Polar Covalent Bond Definitions, Types and Examples What Bond Is Electrons Are Shared Unequally There are two types of covalent bonding: A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Chemists frequently use lewis diagrams to. A covalent bond in which the electrons are shared equally by the two atoms. When electrons are shared between two atoms, they make a. What Bond Is Electrons Are Shared Unequally.
From www.expii.com
Covalent Bonding (Biology) — Definition & Role Expii What Bond Is Electrons Are Shared Unequally It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. Covalent bonds result from the sharing of electrons between atoms. A covalent bond in which. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent. What Bond Is Electrons Are Shared Unequally.
From www.chegg.com
Solved A bond where the electrons are shared unequally, is What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. Chemists frequently use lewis diagrams to. Covalent bonds result from the sharing of electrons between atoms. When electrons. What Bond Is Electrons Are Shared Unequally.
From www.nagwa.com
Question Video Determining How Many Electrons Are Shared in a Single What Bond Is Electrons Are Shared Unequally Covalent bonds result from the sharing of electrons between atoms. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. Chemists frequently use lewis diagrams to. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule.. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Chemical Bonding Chapter ppt download What Bond Is Electrons Are Shared Unequally Chemists frequently use lewis diagrams to. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. A covalent bond in which the electrons are shared equally by the two atoms. There are two types of covalent bonding: Covalent bonds result from the sharing of electrons between atoms.. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513 What Bond Is Electrons Are Shared Unequally It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. A covalent bond in which the electrons are shared equally by the two atoms. There are two types of covalent bonding: When electrons are shared between two atoms, they make a bond. What Bond Is Electrons Are Shared Unequally.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Bond Is Electrons Are Shared Unequally A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Covalent bonds result from the sharing of electrons between atoms. Chemists frequently use lewis diagrams to. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond in which the. What Bond Is Electrons Are Shared Unequally.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Bond Is Electrons Are Shared Unequally A covalent bond in which. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. When electrons are shared between two atoms, they make a bond called a covalent bond. Chemists frequently use lewis diagrams to. There are two types of covalent bonding: It is this sharing. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Covalent bonds where electrons are shared PowerPoint What Bond Is Electrons Are Shared Unequally Covalent bonds result from the sharing of electrons between atoms. A covalent bond in which the electrons are shared equally by the two atoms. Chemists frequently use lewis diagrams to. There are two types of covalent bonding: It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Chemical Bonds ppt download What Bond Is Electrons Are Shared Unequally When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond in which. A covalent bond in which the electrons are shared equally by the two atoms. Covalent bonds result from the sharing of electrons between atoms. Chemists frequently use lewis diagrams to. A molecule that has covalent bonds between its atoms, but. What Bond Is Electrons Are Shared Unequally.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between What Bond Is Electrons Are Shared Unequally A covalent bond in which the electrons are shared equally by the two atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Chemists frequently use lewis diagrams to. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A covalent bond in. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
Molecules EQ How are the electrons arranged in a covalent bond? ppt What Bond Is Electrons Are Shared Unequally A covalent bond in which. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. When electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a. What Bond Is Electrons Are Shared Unequally.
From www.science-revision.co.uk
Electronegativity What Bond Is Electrons Are Shared Unequally A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Covalent bonds result from the sharing of electrons between atoms. A covalent bond in which the electrons are shared equally by the two atoms. It is this sharing of electrons that we refer to as a chemical. What Bond Is Electrons Are Shared Unequally.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Bond Is Electrons Are Shared Unequally A covalent bond in which. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. There are two types of covalent bonding: Chemists frequently use lewis diagrams to. A covalent bond in which the electrons are shared equally by the two atoms. It is this sharing of. What Bond Is Electrons Are Shared Unequally.
From slidetodoc.com
Chemical Bonding Lewis Structures Polarity and Bond Classification What Bond Is Electrons Are Shared Unequally Covalent bonds result from the sharing of electrons between atoms. A covalent bond in which. There are two types of covalent bonding: Chemists frequently use lewis diagrams to. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. A molecule that has covalent bonds between its atoms, but the. What Bond Is Electrons Are Shared Unequally.
From slideplayer.com
HC CHEMISTRY HC CHEMISTRY (B) Periodicity Bonding Continuum. ppt download What Bond Is Electrons Are Shared Unequally A covalent bond in which. Covalent bonds result from the sharing of electrons between atoms. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to. Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in. What Bond Is Electrons Are Shared Unequally.
From www.chegg.com
Solved но H OH н This image is an example of an ionic bond What Bond Is Electrons Are Shared Unequally When electrons are shared between two atoms, they make a bond called a covalent bond. There are two types of covalent bonding: A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Chemists frequently use lewis diagrams to. Covalent bonds result from the sharing of electrons between. What Bond Is Electrons Are Shared Unequally.
From www.slideserve.com
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download What Bond Is Electrons Are Shared Unequally Study with quizlet and memorize flashcards containing terms like which bond term describes a covalent bond in which electrons are shared. When electrons are shared between two atoms, they make a bond called a covalent bond. A molecule that has covalent bonds between its atoms, but the electrons within those bonds are unequally shared, is called a(n) _____ molecule. Chemists. What Bond Is Electrons Are Shared Unequally.