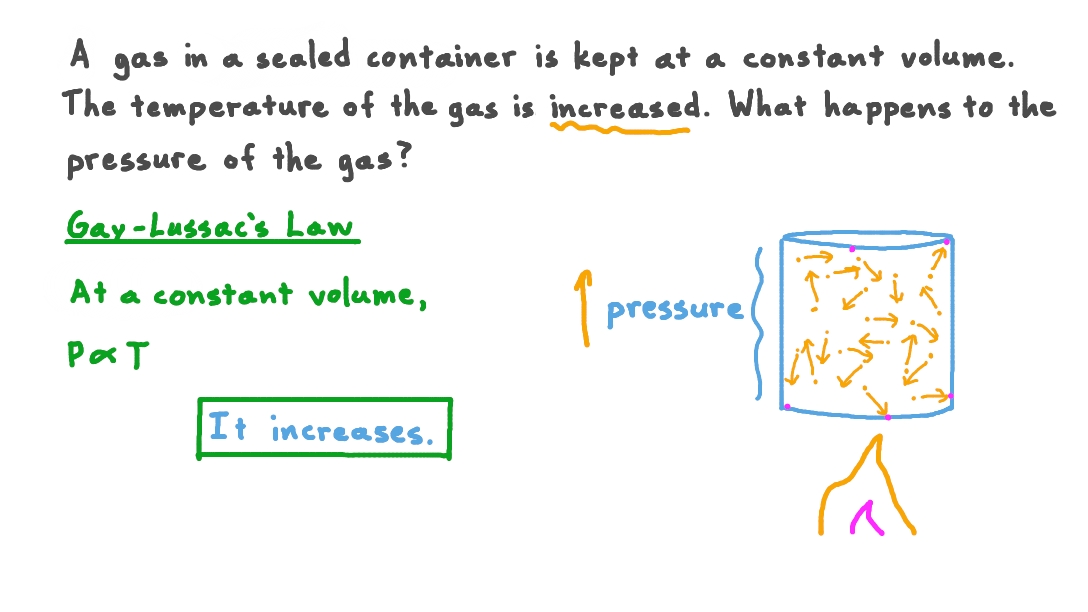
Does Pressure Increase With Temp . This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Volume increases with increasing temperature or amount but. In summary, the conversation discusses the relationship between temperature and pressure in. Modified 4 years, 11 months ago. For example, having water sealed at atmospheric pressure at. Does an increase in pressure necessarily lead to an increase in temperature? As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5.
from www.nagwa.com
Volume increases with increasing temperature or amount but. For example, having water sealed at atmospheric pressure at. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Modified 4 years, 11 months ago. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. In summary, the conversation discusses the relationship between temperature and pressure in. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Does an increase in pressure necessarily lead to an increase in temperature? Yes, at constant density, the pressure increases as the temperature does:
Question Video Determining the Pressure Change of a Gas When Its
Does Pressure Increase With Temp Modified 4 years, 11 months ago. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). For example, having water sealed at atmospheric pressure at. Yes, at constant density, the pressure increases as the temperature does: As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Does an increase in pressure necessarily lead to an increase in temperature? Volume increases with increasing temperature or amount but. Modified 4 years, 11 months ago. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. In summary, the conversation discusses the relationship between temperature and pressure in. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Does Pressure Increase With Temp Modified 4 years, 11 months ago. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. In summary, the conversation discusses the relationship between temperature and pressure in. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature. Does Pressure Increase With Temp.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Does Pressure Increase With Temp Volume increases with increasing temperature or amount but. Yes, at constant density, the pressure increases as the temperature does: Does an increase in pressure necessarily lead to an increase in temperature? The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. For example, having water sealed at atmospheric pressure at. This. Does Pressure Increase With Temp.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Pressure Increase With Temp Does an increase in pressure necessarily lead to an increase in temperature? As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. For example, having water sealed at atmospheric pressure at. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is. Does Pressure Increase With Temp.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download Does Pressure Increase With Temp Does an increase in pressure necessarily lead to an increase in temperature? This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Alternatively, final pressure. Does Pressure Increase With Temp.
From techiescientist.com
Does Density Change With Temperature? Techiescientist Does Pressure Increase With Temp Modified 4 years, 11 months ago. Volume increases with increasing temperature or amount but. In summary, the conversation discusses the relationship between temperature and pressure in. For example, having water sealed at atmospheric pressure at. Does an increase in pressure necessarily lead to an increase in temperature? The relationships among the volume of a gas and its pressure, temperature, and. Does Pressure Increase With Temp.
From exondqbhu.blob.core.windows.net
Gas Pressure Increase With Temperature at William Chandler blog Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. In summary, the conversation discusses the relationship between temperature and pressure in. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Volume increases with increasing temperature or amount but. This. Does Pressure Increase With Temp.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase With Temp As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. For example, having water sealed at atmospheric pressure at. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. This law states that the pressure (p) of a fixed mass of. Does Pressure Increase With Temp.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Does Pressure Increase With Temp In summary, the conversation discusses the relationship between temperature and pressure in. Does an increase in pressure necessarily lead to an increase in temperature? Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Volume increases with increasing temperature or amount but. Yes, at constant density, the pressure increases as the temperature does:. Does Pressure Increase With Temp.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Does Pressure Increase With Temp For example, having water sealed at atmospheric pressure at. Modified 4 years, 11 months ago. Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. As pressure is increased, do we require more energy to increase the temperature from given. Does Pressure Increase With Temp.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Pressure Increase With Temp For example, having water sealed at atmospheric pressure at. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Modified 4 years, 11 months ago. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Volume increases with increasing temperature or. Does Pressure Increase With Temp.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Does Pressure Increase With Temp Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). As pressure is increased, do we require more energy to increase the temperature from given temperature for. Does Pressure Increase With Temp.
From brainly.in
diagram of relation between pressure and temperature Brainly.in Does Pressure Increase With Temp Yes, at constant density, the pressure increases as the temperature does: In summary, the conversation discusses the relationship between temperature and pressure in. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Modified 4 years, 11 months ago. Volume increases with increasing temperature. Does Pressure Increase With Temp.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Modified 4 years, 11 months ago. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. This law states that the pressure (p) of a fixed mass of gas held at. Does Pressure Increase With Temp.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Volume increases with increasing. Does Pressure Increase With Temp.
From www.nagwa.com
Question Video Determining the Pressure Change of a Gas When Its Does Pressure Increase With Temp Volume increases with increasing temperature or amount but. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. For example, having water sealed at atmospheric pressure at. Does an increase in pressure necessarily lead. Does Pressure Increase With Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Does an increase in pressure necessarily lead to an increase in temperature? For example, having water sealed at atmospheric pressure at. In summary, the. Does Pressure Increase With Temp.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). As pressure is increased, do we require more energy to increase the temperature from given. Does Pressure Increase With Temp.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Does Pressure Increase With Temp Does an increase in pressure necessarily lead to an increase in temperature? For example, having water sealed at atmospheric pressure at. Modified 4 years, 11 months ago. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Alternatively, final pressure can be calculated as. Does Pressure Increase With Temp.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID3552130 Does Pressure Increase With Temp Volume increases with increasing temperature or amount but. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Does an increase in pressure necessarily lead to an increase in temperature? For example, having. Does Pressure Increase With Temp.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Does Pressure Increase With Temp For example, having water sealed at atmospheric pressure at. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. Modified 4 years, 11 months ago.. Does Pressure Increase With Temp.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Does Pressure Increase With Temp This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. In summary, the conversation. Does Pressure Increase With Temp.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Does Pressure Increase With Temp Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Yes, at constant density, the pressure increases as the temperature does: In summary, the conversation discusses the relationship between temperature and pressure in. Does an increase in pressure necessarily lead to an increase in temperature? For example, having water sealed at atmospheric pressure. Does Pressure Increase With Temp.
From courses.lumenlearning.com
Phase Changes Physics Does Pressure Increase With Temp In summary, the conversation discusses the relationship between temperature and pressure in. Volume increases with increasing temperature or amount but. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). The relationships among the volume of a gas and its pressure, temperature, and amount. Does Pressure Increase With Temp.
From socratic.org
A given mass of oxygen at room temperature occupies a volume of 500.0 Does Pressure Increase With Temp Does an increase in pressure necessarily lead to an increase in temperature? Yes, at constant density, the pressure increases as the temperature does: Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally. Does Pressure Increase With Temp.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Increase With Temp Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. For example, having water sealed at atmospheric pressure at. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Does an increase in pressure necessarily lead to. Does Pressure Increase With Temp.
From www.slideshare.net
Air pressure Does Pressure Increase With Temp This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. For example, having water sealed at atmospheric pressure at. As pressure is increased, do we require more. Does Pressure Increase With Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Temp The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. For example, having water sealed at atmospheric pressure at. Modified 4 years, 11 months ago. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Yes, at constant density, the pressure increases as the. Does Pressure Increase With Temp.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase With Temp Modified 4 years, 11 months ago. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in. Does Pressure Increase With Temp.
From socratic.org
How do mountains affect temperature on both the windward side and the Does Pressure Increase With Temp As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). For example, having water sealed at atmospheric pressure at. The relationships among the volume. Does Pressure Increase With Temp.
From klaezjnuy.blob.core.windows.net
Pressure Temperature Chart Solid Liquid Gas at Renee Thomas blog Does Pressure Increase With Temp In summary, the conversation discusses the relationship between temperature and pressure in. For example, having water sealed at atmospheric pressure at. Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. Volume increases with increasing temperature or amount but. Modified 4 years, 11 months ago. This law states that the pressure (p) of. Does Pressure Increase With Temp.
From klatordwr.blob.core.windows.net
Does Pressure Rise With Temperature at Eric Thomson blog Does Pressure Increase With Temp Modified 4 years, 11 months ago. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). Alternatively, final pressure can be calculated as initial. Does Pressure Increase With Temp.
From pediaa.com
Relationship Between Pressure and Temperature Does Pressure Increase With Temp This law states that the pressure (p) of a fixed mass of gas held at a constant volume is directionally proportional to its kelvin temperature (t). As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. The relationships among the volume of a gas and its pressure, temperature, and amount. Does Pressure Increase With Temp.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Pressure Increase With Temp Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. For example, having water sealed at atmospheric pressure at. Yes, at constant density, the pressure increases as the temperature does: Volume increases with increasing temperature or amount but. Does an increase in pressure necessarily lead to an increase in temperature? Modified 4 years,. Does Pressure Increase With Temp.
From slideplayer.com
Density. ppt download Does Pressure Increase With Temp Modified 4 years, 11 months ago. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure 6.3.5. As pressure is increased, do we require more energy to increase the temperature from given temperature for the same mass. This law states that the pressure (p) of a fixed mass of gas held at. Does Pressure Increase With Temp.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Increase With Temp Does an increase in pressure necessarily lead to an increase in temperature? Alternatively, final pressure can be calculated as initial pressure multiplied by final temperature divided by initial temperature. In summary, the conversation discusses the relationship between temperature and pressure in. Yes, at constant density, the pressure increases as the temperature does: Volume increases with increasing temperature or amount but.. Does Pressure Increase With Temp.