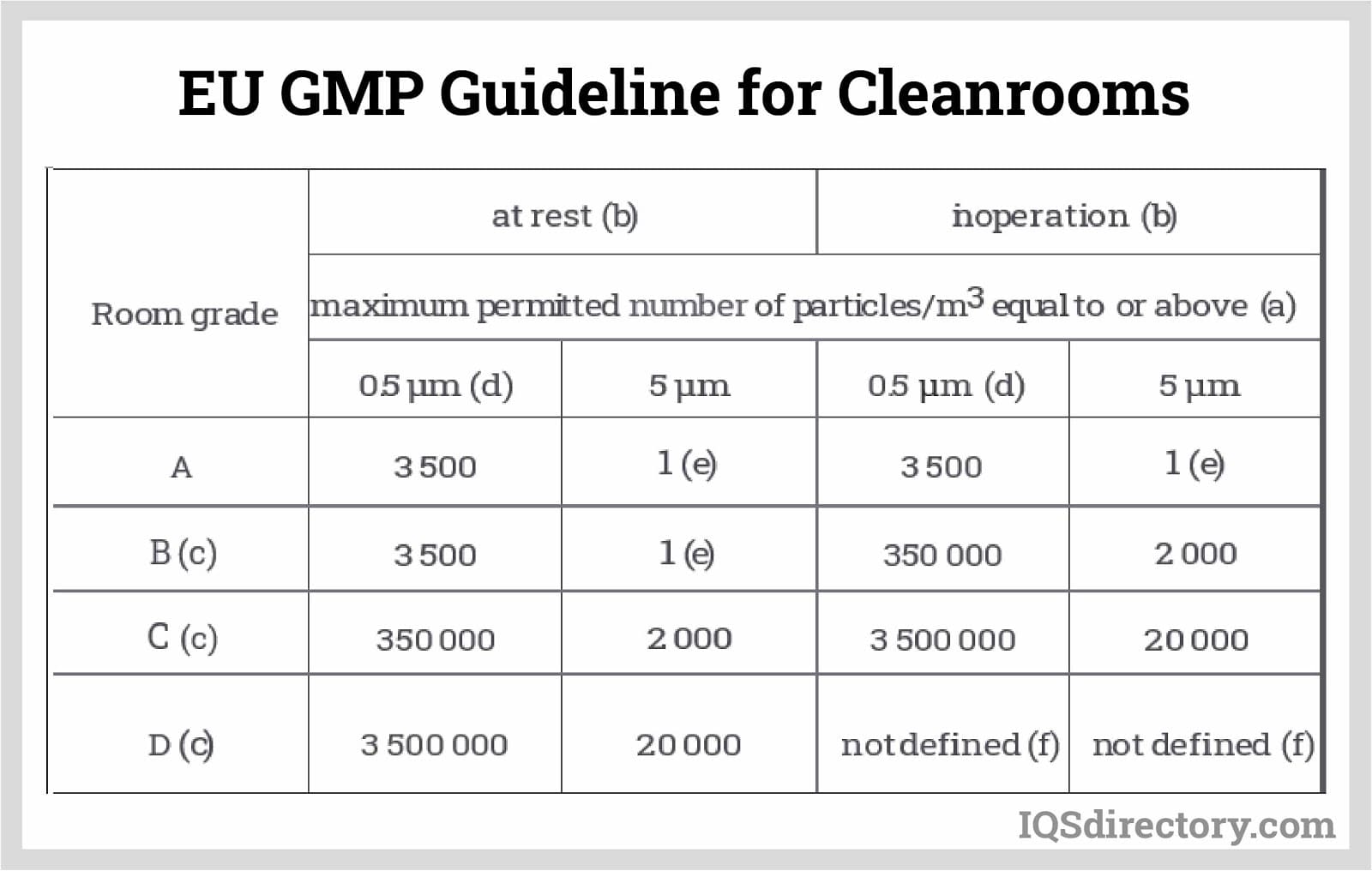
Clean Room Gmp . So why do i need a clean room? A, b, c, d for contamination control in pharmaceuticals. Here is 8 gmp cleanroom requirements you need for your gmp. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This will help you design your gmp cleanroom facility. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. What makes a gmp facility ‘’gmp’’? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. How do they differ from ‘’regular’’ cleanrooms? In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. Understand air cleanliness & particle counts for compliance.
from www.iqsdirectory.com
So why do i need a clean room? This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. How do they differ from ‘’regular’’ cleanrooms? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. Here is 8 gmp cleanroom requirements you need for your gmp. Understand air cleanliness & particle counts for compliance. A, b, c, d for contamination control in pharmaceuticals. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e.
Cleanroom What is it? ISO Standards and Classifications, Design, Types
Clean Room Gmp When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. So why do i need a clean room? What makes a gmp facility ‘’gmp’’? Understand air cleanliness & particle counts for compliance. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This will help you design your gmp cleanroom facility. Here is 8 gmp cleanroom requirements you need for your gmp. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. How do they differ from ‘’regular’’ cleanrooms? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. A, b, c, d for contamination control in pharmaceuticals.
From gmptech.net
GMP Technical Solutions Clean Room Gmp Understand air cleanliness & particle counts for compliance. When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. Here is 8 gmp cleanroom requirements you need for your gmp. This. Clean Room Gmp.
From angstromtechnology.com
What Industries Require GMP Cleanrooms? Angstrom Technology Clean Room Gmp A, b, c, d for contamination control in pharmaceuticals. This will help you design your gmp cleanroom facility. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. How do they differ from ‘’regular’’ cleanrooms? So why do i need a clean room? What makes. Clean Room Gmp.
From allcells.com
AllCells Announces New GMP Cleanroom AllCells Clean Room Gmp When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. Understand air cleanliness & particle counts for compliance. So why do i need a clean room? Here is 8 gmp. Clean Room Gmp.
From www.sctcleanroom.com
News WHAT CONTENT ARE INCLUDED IN GMP CLEAN ROOM STANDARDS? Clean Room Gmp This will help you design your gmp cleanroom facility. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. So why do i need a clean room? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. In a pharmaceutical. Clean Room Gmp.
From nicomac.com
Design build validation of Modular Cleanrooms Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This will help you design your gmp cleanroom facility. How do they differ from ‘’regular’’ cleanrooms? What makes a gmp facility ‘’gmp’’? So. Clean Room Gmp.
From shanghai-marya.en.made-in-china.com
China GMP/ISO PU Sandwich Panel Clean Room System Project for Clean Room Gmp So why do i need a clean room? What makes a gmp facility ‘’gmp’’? A, b, c, d for contamination control in pharmaceuticals. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d.. Clean Room Gmp.
From www.zonsteel.com
GMP Clean Room Expert Manufacturer and Supplier Zonsteel Clean Room Gmp This will help you design your gmp cleanroom facility. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. A, b, c, d for contamination control in pharmaceuticals. How do they differ from ‘’regular’’ cleanrooms? This article simplifies the understanding of the gmp cleanroom requirements. Clean Room Gmp.
From oms-technology.com
Pharmaceutical GMP Cleanroom Biosafety laboratory design Clean Room Gmp This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. Understand air cleanliness & particle counts for compliance. What makes a gmp facility ‘’gmp’’? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. A, b, c, d for contamination. Clean Room Gmp.
From www.youtube.com
GMP Clean Room for Desiccant Automated Machines YouTube Clean Room Gmp This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. So why do i need a clean room? This will help you design your gmp cleanroom facility. Here is 8 gmp cleanroom requirements you need for your gmp. When utilised for critical activities, they are required to meet the contamination. Clean Room Gmp.
From www.mecart-cleanrooms.com
GMP Class B Sterile Operation Cleanroom MECART Cleanrooms Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. How do they differ from ‘’regular’’ cleanrooms? A, b, c, d for contamination control in pharmaceuticals. Understand air. Clean Room Gmp.
From www.sctcleanroom.com
News HOW TO DO A GMP CLEAN ROOM? & HOW TO CALCULATE AIR CHANGE? Clean Room Gmp Understand air cleanliness & particle counts for compliance. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This will help you design your gmp cleanroom facility. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. What. Clean Room Gmp.
From www.zonsteel.com
GMP Clean Room Expert Manufacturer and Supplier Zonsteel Clean Room Gmp What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. How do they differ from ‘’regular’’ cleanrooms? So why do i need a clean room? Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by. Clean Room Gmp.
From www.researchgate.net
Clean room as a facility with controlled temperature Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. What makes a gmp facility ‘’gmp’’? How do they differ from ‘’regular’’ cleanrooms? Here is 8 gmp cleanroom requirements you need. Clean Room Gmp.
From www.cleanroom-industries.com
GMP in Cleanroom Maintenance Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. A, b, c, d for contamination control in pharmaceuticals. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. In a pharmaceutical sense, clean rooms are those rooms that meet the. Clean Room Gmp.
From www.mybeckman.uk
MET ONE 3400+ Air Particle Counter GMP Cleanroom Routine Clean Room Gmp This will help you design your gmp cleanroom facility. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. A, b, c, d for contamination control in pharmaceuticals. How do they differ from ‘’regular’’ cleanrooms? What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification and routine environmental. Clean Room Gmp.
From www.iskra-pio.si
SmartCon GMP Clean rooms Iskra PIO Clean Room Gmp Here is 8 gmp cleanroom requirements you need for your gmp. A, b, c, d for contamination control in pharmaceuticals. How do they differ from ‘’regular’’ cleanrooms? This will help you design your gmp cleanroom facility. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. What makes. Clean Room Gmp.
From www.cleanroom-industries.com
GMP Compliant Cleanroom Clean Room Gmp This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This will help you design your gmp cleanroom facility. A, b, c, d for contamination control in pharmaceuticals. What makes a gmp facility. Clean Room Gmp.
From www.cleanroomswest.com
Cell Therapy GMP Production Suites Clean Rooms West, Inc. Clean Room Gmp This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. What makes a gmp facility ‘’gmp’’? So why do i need a clean room? This will help you design your gmp cleanroom facility. When utilised for critical activities, they are required to meet the contamination control requirements of an eu. Clean Room Gmp.
From www.mecart-cleanrooms.com
Building a GMP Facility 8 GMP Cleanroom Requirements MECART Clean Room Gmp Understand air cleanliness & particle counts for compliance. Here is 8 gmp cleanroom requirements you need for your gmp. So why do i need a clean room? How do they differ from ‘’regular’’ cleanrooms? A, b, c, d for contamination control in pharmaceuticals. When utilised for critical activities, they are required to meet the contamination control requirements of an eu. Clean Room Gmp.
From angstromtechnology.com
What Are GMP Standards for Cleanrooms? Angstrom Technology Clean Room Gmp Here is 8 gmp cleanroom requirements you need for your gmp. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. So why do i need a clean room? Understand air cleanliness & particle counts for compliance. When utilised for critical activities, they are required to meet the contamination control requirements of an. Clean Room Gmp.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. So why do i need a clean room? Understand air cleanliness & particle counts for compliance. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. When utilised. Clean Room Gmp.
From www.einsteinsinc.com
Clean Room Construction GMP / GxP Einstein's Solutions Clean Room Gmp A, b, c, d for contamination control in pharmaceuticals. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This will help you design your gmp cleanroom facility. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. Understand air cleanliness. Clean Room Gmp.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Clean Room Gmp So why do i need a clean room? What makes a gmp facility ‘’gmp’’? Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This will help you design your gmp. Clean Room Gmp.
From www.phchd.com
ISO and GMP Cleanroom Standards PHCbi Clean Room Gmp A, b, c, d for contamination control in pharmaceuticals. Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. This paper discusses the. Clean Room Gmp.
From spraycare.com.tw
GMP Class 100,000 Clean Room 福禹達生技股份有限公司 Clean Room Gmp Here is 8 gmp cleanroom requirements you need for your gmp. This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This will help you design your gmp cleanroom facility. Annex 1 of. Clean Room Gmp.
From www.zonsteel.com
GMP Clean Room Expert Manufacturer and Supplier Zonsteel Clean Room Gmp Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. What makes a gmp facility ‘’gmp’’? This will help you design your gmp cleanroom facility. When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. In a pharmaceutical. Clean Room Gmp.
From www.cbmmaryland.com
GMP Cleaning Procedures Commercial Building Maintenance Clean Room Gmp So why do i need a clean room? A, b, c, d for contamination control in pharmaceuticals. When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman. Clean Room Gmp.
From www.cleanroom-industries.com
Designs for GMP Clean Rooms Clean Room Gmp So why do i need a clean room? Annex 1 of both the eu and pic/s guides to gmp and other standards and guidance as required by local health authorities. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. This will help you design. Clean Room Gmp.
From www.pinterest.com.mx
GMP Cleanrooms; some of the world's best brands rely on GMP Cleanrooms Clean Room Gmp A, b, c, d for contamination control in pharmaceuticals. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. Understand air cleanliness & particle counts for compliance. Annex 1 of. Clean Room Gmp.
From www.cleanroom-fab.com
GMP Sterile Aseptic ISO Laboratory Clean Rooms Physical Chemical Clean Room Gmp In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification. Clean Room Gmp.
From oms-technology.com
Pharmaceutical GMP Cleanroom Biosafety laboratory design Clean Room Gmp Understand air cleanliness & particle counts for compliance. How do they differ from ‘’regular’’ cleanrooms? This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. This will help you design your gmp cleanroom facility. So why do i need a clean room? Here is 8 gmp cleanroom requirements you need. Clean Room Gmp.
From atlanticbuildingsolutions.com
Cleanroom Janitorial Services & GMP Cleaning Atlantic Building Solutions Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. So why do i need a clean room? When utilised for critical activities, they are required to meet the contamination control requirements of an eu ggmp grade a. What makes a gmp facility ‘’gmp’’? Annex 1 of both the eu and pic/s guides. Clean Room Gmp.
From nicomac.com
Design build validation of Modular Cleanrooms Clean Room Gmp This article simplifies the understanding of the gmp cleanroom requirements for grade a, b, c, and d. Understand air cleanliness & particle counts for compliance. This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. So why do i need a clean room? Here is 8 gmp cleanroom requirements you. Clean Room Gmp.
From www.cleanroom-fab.com
GMP ISO Clean Rooms For Medical Devices Clean Room Gmp How do they differ from ‘’regular’’ cleanrooms? A, b, c, d for contamination control in pharmaceuticals. Understand air cleanliness & particle counts for compliance. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. This will help you design your gmp cleanroom facility. So why. Clean Room Gmp.
From www.isocleanroomchina.com
GMP Clean Room Manufacturer in China Sunnyda Clean Room Gmp Understand air cleanliness & particle counts for compliance. In a pharmaceutical sense, clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp, i.e. What makes a gmp facility ‘’gmp’’? This paper discusses the differences between gmp cleanroom classification and routine environmental monitoring and explains how beckman coulter can help. When. Clean Room Gmp.