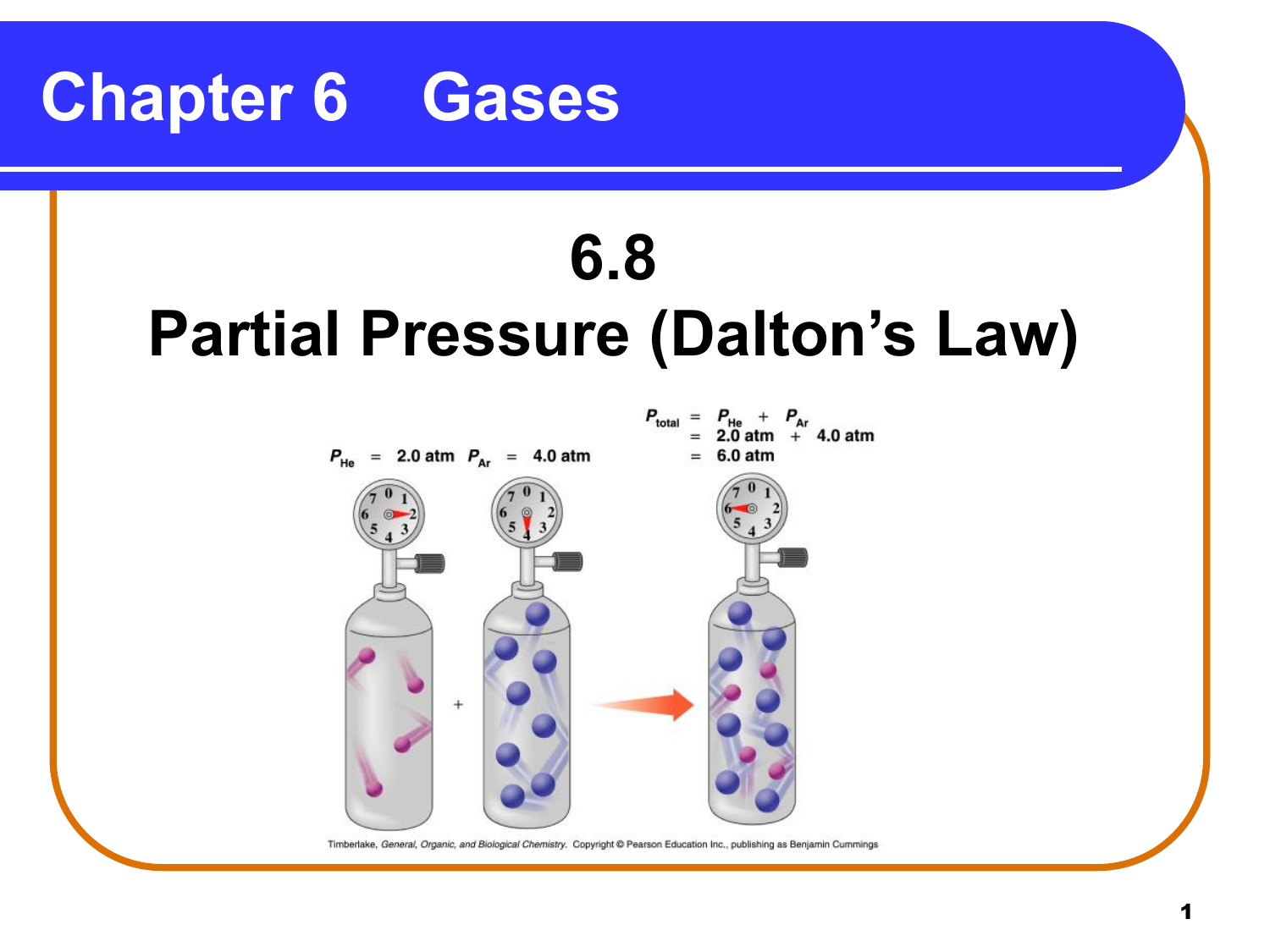
Does Temperature Increase Partial Pressure . The pressure exerted by each gas in a gas mixture (its partial. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. In this scenario, the air is not confined. The partial pressure of a gas is the pressure that gas would. The partial pressure of each gas in a mixture is proportional to its mole fraction. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask.
from studylib.net
In this scenario, the air is not confined. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The pressure exerted by each gas in a gas mixture (its partial. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. The partial pressure of a gas is the pressure that gas would. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The partial pressure of each gas in a mixture is proportional to its mole fraction.
Partial Pressure
Does Temperature Increase Partial Pressure The pressure exerted by each gas in a gas mixture (its partial. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The partial pressure of a gas is the pressure that gas would. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. The partial pressure of each gas in a mixture is proportional to its mole fraction. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The pressure exerted by each gas in a gas mixture (its partial. In this scenario, the air is not confined. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Temperature Increase Partial Pressure At higher altitudes, the temperature is generally lower, and the air pressure is also lower. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the. Does Temperature Increase Partial Pressure.
From derangedphysiology.com
Partial pressure and the solubility of gases in biological systems Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. In this scenario, the air is not confined. The partial pressure of a gas is the pressure that gas would. The pressure exerted by each gas in a gas mixture (its partial. At any temperature, the partial pressure of. Does Temperature Increase Partial Pressure.
From www.slideserve.com
PPT 12.2 DALTON’S LAW OF PARTIAL PRESSURES PowerPoint Presentation Does Temperature Increase Partial Pressure The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the. Does Temperature Increase Partial Pressure.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube Does Temperature Increase Partial Pressure The partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure exerted by each gas in a gas mixture (its partial. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. In this scenario, the air is not. Does Temperature Increase Partial Pressure.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Does Temperature Increase Partial Pressure In this scenario, the air is not confined. The partial pressure of each gas in a mixture is proportional to its mole fraction. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The. Does Temperature Increase Partial Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Temperature Increase Partial Pressure The pressure exerted by each gas in a gas mixture (its partial. In this scenario, the air is not confined. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. At any temperature, the partial pressure of the water in the air cannot exceed. Does Temperature Increase Partial Pressure.
From breathe.ersjournals.com
Relating oxygen partial pressure, saturation and content the Does Temperature Increase Partial Pressure At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure exerted by each gas in a gas mixture (its partial. The vapor. Does Temperature Increase Partial Pressure.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. In this scenario, the air is not confined. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. At higher altitudes, the temperature is generally lower, and the air. Does Temperature Increase Partial Pressure.
From www.youtube.com
How to Calculate Partial Pressure and Total Pressure Using Dalton's Law Does Temperature Increase Partial Pressure In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The partial pressure of a gas is the pressure that gas would. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever. Does Temperature Increase Partial Pressure.
From pressbooks.bccampus.ca
Unit 4 The Respiratory System Douglas College Human Anatomy Does Temperature Increase Partial Pressure At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. The partial pressure of a gas is the pressure that gas would. The partial pressure of each gas in a mixture is proportional to its. Does Temperature Increase Partial Pressure.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Does Temperature Increase Partial Pressure The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. At any temperature, the partial pressure of the water in the air. Does Temperature Increase Partial Pressure.
From slideplayer.com
Gases Gasses ppt download Does Temperature Increase Partial Pressure The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. The pressure exerted by each gas in a gas mixture (its partial. In this scenario, the air is not confined. The partial pressure of a gas is the pressure that gas would. The partial pressure of each gas in a. Does Temperature Increase Partial Pressure.
From www.researchgate.net
Alterations of partial pressure in the heat exchanger. Download Does Temperature Increase Partial Pressure At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. In this scenario, the air is not confined. The partial pressure of a gas is the pressure that gas would. The partial pressure of. Does Temperature Increase Partial Pressure.
From www.youtube.com
6.05 Dalton's Law of Partial Pressures YouTube Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. In this scenario, the air is not confined. The pressure exerted by each gas in a gas mixture (its partial. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the. Does Temperature Increase Partial Pressure.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Temperature Increase Partial Pressure At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The partial pressure. Does Temperature Increase Partial Pressure.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. The vapor pressure of a substance depends on. Does Temperature Increase Partial Pressure.
From mungfali.com
Partial Pressure Diagram Does Temperature Increase Partial Pressure The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The partial pressure of a gas is the pressure that gas would. The partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure of water vapour. Does Temperature Increase Partial Pressure.
From byjus.com
Partial Pressure Formula, Dalton's Law, Mixture of Ideal Gas, Examples Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever the partial pressure reaches the vapor. The partial pressure of a gas is the. Does Temperature Increase Partial Pressure.
From rdreview.jaea.go.jp
Fig.132 Calculated phase diagram of temperature vs. oxygen partial Does Temperature Increase Partial Pressure The pressure exerted by each gas in a gas mixture (its partial. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure. Does Temperature Increase Partial Pressure.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Does Temperature Increase Partial Pressure The partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because. Does Temperature Increase Partial Pressure.
From ditki.com
Physiology Glossary Partial Pressures ditki medical & biological Does Temperature Increase Partial Pressure The partial pressure of a gas is the pressure that gas would. The partial pressure of each gas in a mixture is proportional to its mole fraction. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. This law states that in a mixture of two or more gases, the. Does Temperature Increase Partial Pressure.
From www.youtube.com
Example Computing partial pressures and enthalpy change in a mixture Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. This law states that in a mixture of two or more. Does Temperature Increase Partial Pressure.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID64297 Does Temperature Increase Partial Pressure In this scenario, the air is not confined. This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The partial pressure of each gas in a mixture is proportional to its mole fraction. The pressure of water vapour is constant at 47 mmhg at. Does Temperature Increase Partial Pressure.
From www.researchgate.net
Effect of reaction temperature and butanol partial pressure on the most Does Temperature Increase Partial Pressure In this scenario, the air is not confined. The partial pressure of a gas is the pressure that gas would. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The pressure exerted by each gas in a gas mixture (its partial. The vapor pressure of a substance depends. Does Temperature Increase Partial Pressure.
From www.youtube.com
Henry's Law Partial pressure, gas solubility, and mole fraction YouTube Does Temperature Increase Partial Pressure The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. The partial pressure of each gas in a mixture is proportional to its mole fraction. At any temperature, the partial pressure of the water in the air cannot exceed the vapor pressure of the water at that temperature, because whenever. Does Temperature Increase Partial Pressure.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Does Temperature Increase Partial Pressure At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The partial pressure of each gas in a mixture is proportional to its mole fraction. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The partial. Does Temperature Increase Partial Pressure.
From www.doubtnut.com
Solubility of oxygen gas in water follows Henry's law. When the Does Temperature Increase Partial Pressure In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The pressure exerted by each gas in a gas mixture (its partial. The partial pressure of. Does Temperature Increase Partial Pressure.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Does Temperature Increase Partial Pressure This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. At higher altitudes, the temperature is generally lower, and the air pressure is. Does Temperature Increase Partial Pressure.
From breathe.ersjournals.com
Relating oxygen partial pressure, saturation and content the Does Temperature Increase Partial Pressure The partial pressure of a gas is the pressure that gas would. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each. Does Temperature Increase Partial Pressure.
From studylib.net
Partial Pressure Does Temperature Increase Partial Pressure The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The partial pressure of each gas in a mixture is proportional to its mole fraction. The partial pressure of a gas is the pressure that gas would. In this scenario, the air. Does Temperature Increase Partial Pressure.
From www.researchgate.net
Temperature dependence of the equilibrium oxygen partial pressure for Does Temperature Increase Partial Pressure The pressure exerted by each gas in a gas mixture (its partial. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the. Does Temperature Increase Partial Pressure.
From www.youtube.com
What is partial pressure? YouTube Does Temperature Increase Partial Pressure In this scenario, the air is not confined. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The vapor pressure of a substance depends on both the substance and its temperature—an increase in temperature increases the vapor. This law states that. Does Temperature Increase Partial Pressure.
From byjus.com
how partial pressure depends upon temperature explain in detail Does Temperature Increase Partial Pressure This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The partial pressure of a gas is the pressure that gas would. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. Does Temperature Increase Partial Pressure.
From philschatz.com
Transport of Gases · Anatomy and Physiology Does Temperature Increase Partial Pressure The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. In this scenario, the air is not confined. The partial pressure of each gas in a mixture is proportional to its mole fraction. The partial pressure of a gas is the pressure that gas would. The vapor pressure of. Does Temperature Increase Partial Pressure.
From www.researchgate.net
Partial Pressure of water vapor vs. temperature, including saturation Does Temperature Increase Partial Pressure This law states that in a mixture of two or more gases, the total pressure is the sum of the partial pressures of all the components. The pressure of water vapour is constant at 47 mmhg at normal body temperature (37°c), and it is strongly temperature dependent. The partial pressure of each gas in a mixture is proportional to its. Does Temperature Increase Partial Pressure.